Preparation of alkaline anion exchange membrane fuel cell electrode catalysis layer three-dimensional resin
A fuel cell electrode, three-dimensional resin technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problem of large steric hindrance, reduced polymer molecular chain force, resin adhesion and mechanical strength can not guarantee fuel cells Long-term operation and other problems, to achieve the effect of high ion exchange membrane capacity, good chemical stability and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve 1 weight unit of polyarylsulfone (PASF) in 10 weight units of 1,1,2,2-tetrachloroethane, add 1 weight unit of anhydrous zinc chloride, and then add 20 weight units of 1, 4-dichloromethoxybutane, stirred and reacted at 40°C for 4 hours, poured the reaction solution into ethanol, filtered, and dried to obtain the chloromethylated polymer product, the chloromethylated polymer was Soak in 33wt% trimethylamine aqueous solution for 12 hours at ℃, take out, soak in 0.1M KOH aqueous solution for 12 hours at 30 ℃, polymer is washed to neutrality with deionized water, obtains alkalized polymer (QAPASF-OH ).
[0023] The ion exchange capacity of the product was 1.75 mmol / g.
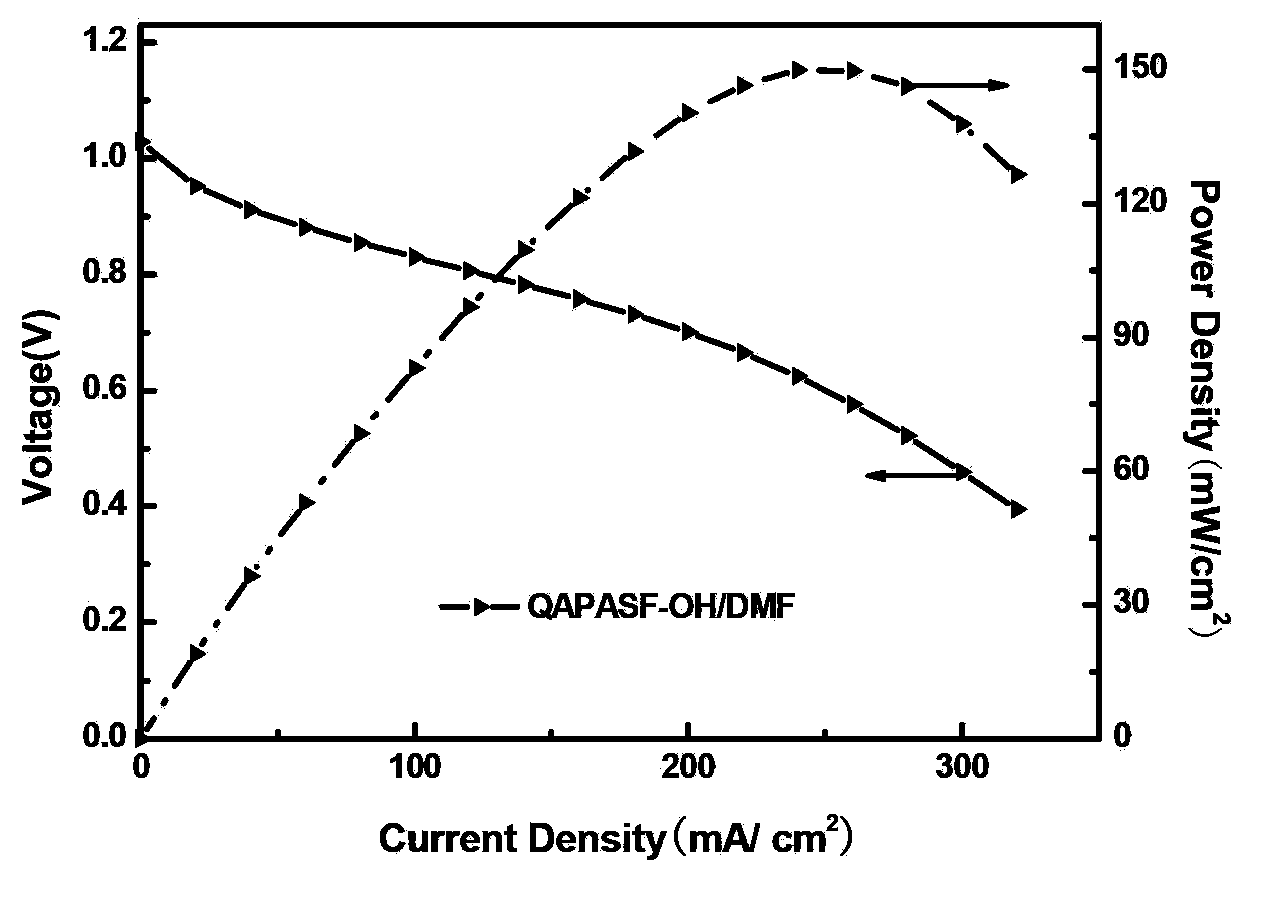
[0024] The alkalinized polymer was dissolved in N,N-dimethylformamide (DMF) to make a 2wt% solution to obtain the three-dimensional resin of the QAPASF-OH / DMF electrode catalytic layer.
[0025] H using QAPASF-OH / DMF stereoresin 2 / O 2 fuel cell performance figure 1 As shown, it can be seen that...
Embodiment 2
[0027] Dissolve 1 weight unit of bisphenol A polyethersulfone (PSf) in 11 weight units of 1,1-dichloroethane, add 1.1 weight units of anhydrous aluminum chloride, and then add 21 weight units of 1, 4-dichloromethoxybutane, stirred and reacted at 50°C for 5 hours, poured the reaction solution into ethanol, filtered, and dried to obtain a chloromethylated polymer product, and prepared chlorine at 30°C The methylated product was soaked in triethylamine for 24 hours, taken out, soaked in 0.2M KOH aqueous solution at 35°C for 20 hours, and rinsed with deionized water until neutral to obtain an alkalized polymer (QAPSf-OH ).
[0028] The ion exchange capacity of the product was 1.61 mmol / g.
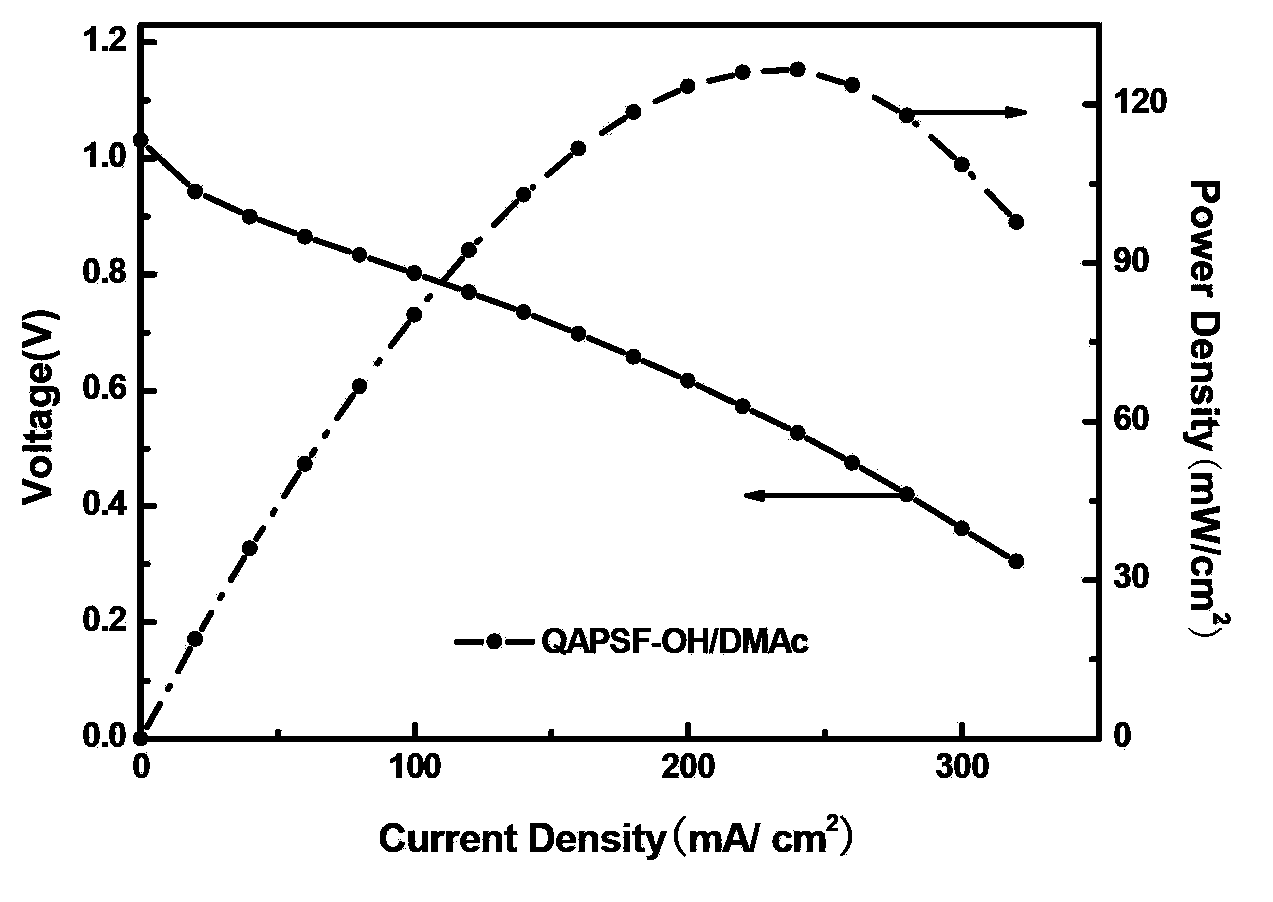
[0029] The alkalinized polymer was dissolved in N,N-dimethylacetamide (DMAc) to make a 3wt% solution to obtain the three-dimensional resin of the QAPSf-OH / DMAc electrode catalytic layer.
[0030] H using QAPSf-OH / DMAc stereoresin 2 / O 2 fuel cell performance figure 2 As shown, its highest...
Embodiment 3
[0032] Dissolve 1 weight unit of polyether sulfone ketone (PPESK) in 12 weight units of 1,2-dichloroethane, add 1.2 weight units of anhydrous tin chloride, and then add 22 weight units of 1 , 4-dichloromethoxybutane, stirred and reacted at 60°C for 6 hours, poured the reaction solution into ethanol, filtered, dried to obtain chloromethylated polymer product, soaked the above product at 40°C In tripropylamine for 30 hours, take it out, soak it in 0.5M KOH aqueous solution at 40°C for 24 hours, wash the polymer with deionized water until it is neutral, and obtain an alkalized polymer (QAPPESK-OH).
[0033] The ion exchange capacity of the product was 1.55 mmol / g.
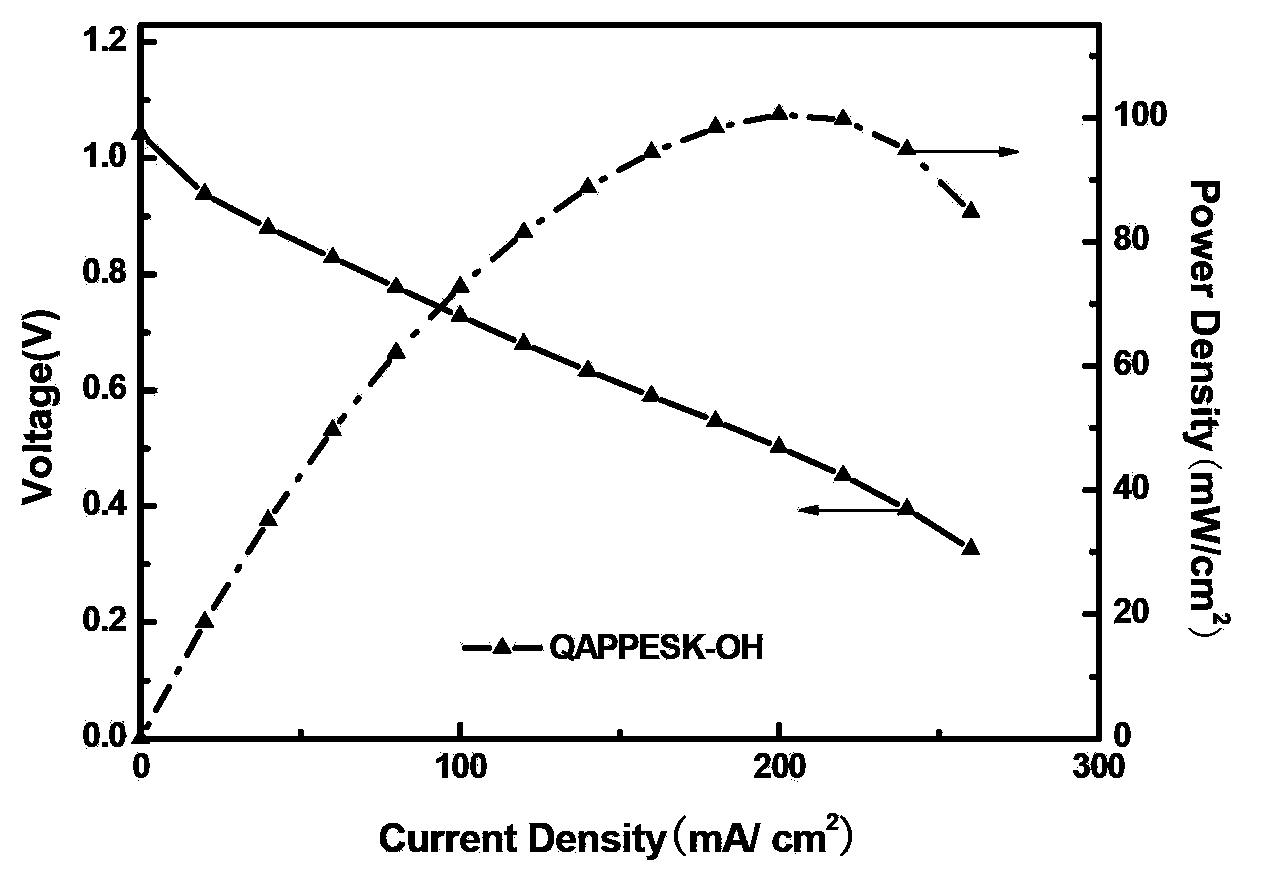
[0034] The alkalized polymer is dissolved in a mixed solvent of water and ethanol (40:60 in volume ratio) to make a 4wt% solution to obtain the three-dimensional resin of the QAPPESK-OH type electrode catalyst layer.
[0035] H using QAPPESK-OH stereoresin 2 / O 2 fuel cell performance image 3 As shown, its highes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com