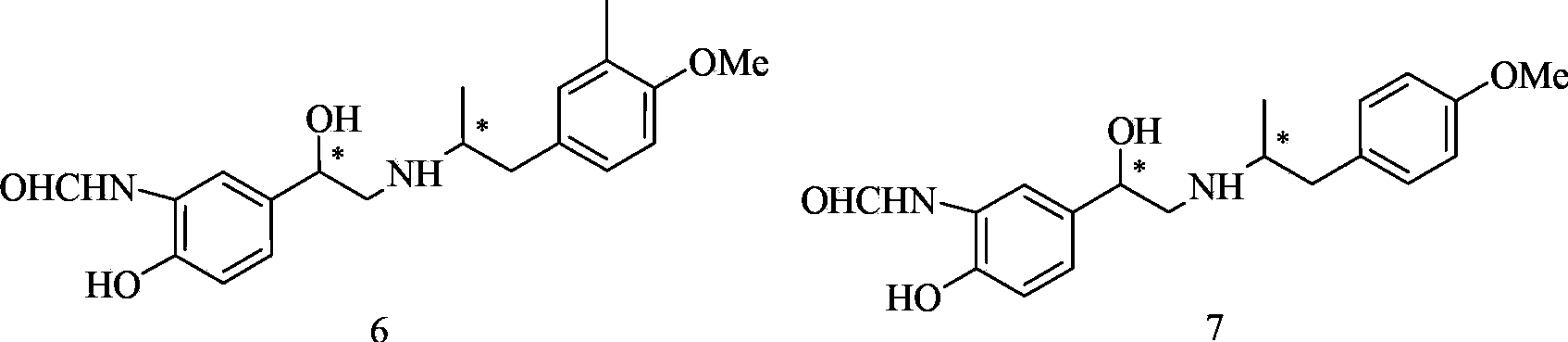
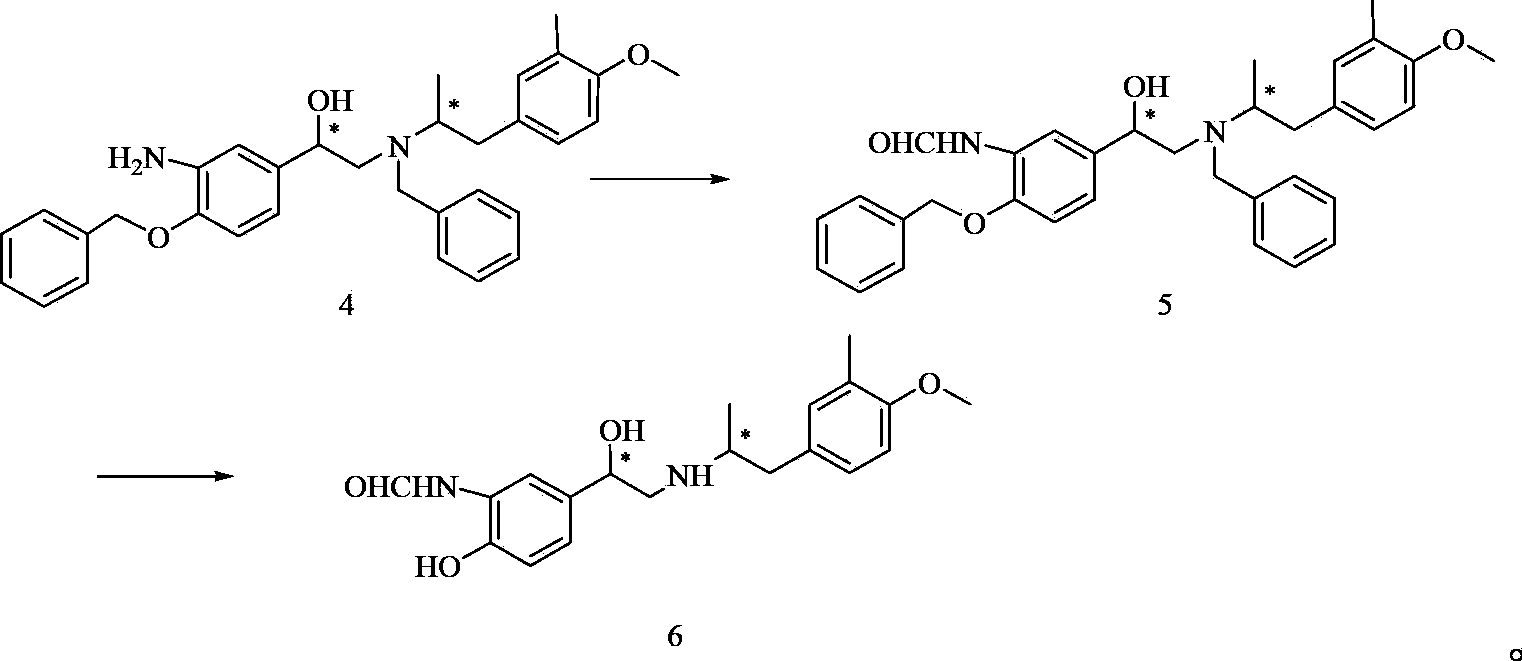
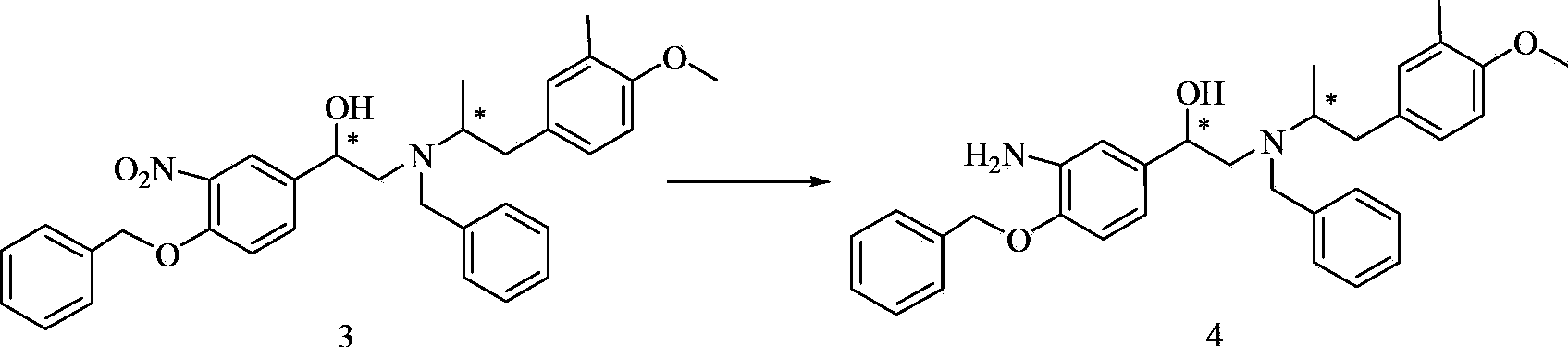
Methanamide compound, preparation method of intermediate of methanamide compound, and applications of the intermediate
A formamide compound and compound technology, which is applied in the preparation of organic compounds, amino hydroxyl compounds, carboxylic acid amides, etc., can solve the problems such as no report on the synthesis of formamide compound 6
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0169] 2-[N-Benzyl-N-[1-methyl-2-(4-methoxy-3-chloromethylphenyl)]ethyl]amino-1-(4-benzyloxy-3- Preparation of nitrophenyl)ethanone (compound 2, * in the structural formula represents the achiral central carbon atom, that is, compound 2 is a racemate)
[0170] In a 25mL four-neck flask, add 1g of compound 1 (compound 1, * in the structural formula represents the achiral central carbon atom, that is, compound 1 is a racemate, CAS: 43229-66-9), 10mL of anhydrous acetic acid, and stir After complete dissolution, add 2mL of 35% formaldehyde aqueous solution, 4mL of concentrated hydrochloric acid and 0.05g of anhydrous zinc chloride at one time, react at 90°C for 0.5h, and TLC detects that the raw material point disappears.
[0171] Under ice-water bath cooling, use 5mol / L NaOH aqueous solution to adjust the pH of the reaction solution to 9-11, extract three times with 20mL ethyl acetate, separate the organic layer, wash once with 30mL water, wash three times with 20mL saturated sa...
Embodiment 2
[0174] 2-[N-Benzyl-N-[1-methyl-2-(4-methoxy-3-chloromethylphenyl)]ethyl]amino-1-(4-benzyloxy-3- Preparation of nitrophenyl)ethanone (compound 2, * in the structural formula represents the achiral central carbon atom, that is, compound 2 is a racemate)
[0175] In a 100mL four-neck flask, add 4g of compound 1, 40mL of anhydrous acetic acid, stir to dissolve completely, add 8mL of 35% formaldehyde solution, 16mL of concentrated hydrochloric acid and 0.4g of anhydrous zinc chloride at one time, and react at 85°C for 1h. TLC detects that the raw material spot disappears.
[0176] Under ice-water bath cooling, use 5mol / L NaOH aqueous solution to adjust the pH of the reaction solution to 9-11, extract three times with 100mL ethyl acetate, separate the organic layer, wash once with 100mL water, wash three times with 200mL saturated saline, and anhydrous Dry over sodium sulfate and concentrate to give 4.3 g of crude solid. Recrystallized with ethanol / acetone to obtain 1.7 g of compo...
Embodiment 3
[0178] 2-[N-Benzyl-N-[1-methyl-2-(4-methoxy-3-chloromethylphenyl)]ethyl]amino-1-(4-benzyloxy-3- Preparation of nitrophenyl)ethanone (compound 2, * in the structural formula represents the achiral central carbon atom, that is, compound 2 is a racemate)
[0179] In a 500mL four-neck flask, add 18g of compound 1, 180mL of anhydrous acetic acid, stir to dissolve completely, add 36mL of 35% formaldehyde solution, 72mL of concentrated hydrochloric acid and 3.6g of anhydrous zinc chloride at one time, and react at 60°C for 2 hours. TLC detects that the raw material spot disappears.
[0180] Under ice-water bath cooling, use 5mol / L NaOH aqueous solution to adjust the pH of the reaction solution to 9-11, extract three times with 400mL ethyl acetate, separate the organic layer, wash once with 400mL water, wash three times with 300mL saturated saline, anhydrous Dry over sodium sulfate and concentrate to obtain 20.1 g of crude solid. Recrystallized with ethanol / ethyl acetate = 10 / 1 to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com