Entecavir compound nano-preparation and preparation method thereof
A kind of entecavir and nano-composite technology, which is applied in antiviral agents, pharmaceutical formulas, active ingredients of heterocyclic compounds, etc., can solve the problems of adverse reactions such as gastrointestinal irritation, low utilization rate of entecavir, and difficulty in controlling drug efficacy, and achieve reduction Adverse reactions and gastrointestinal irritation, solving poor bioavailability, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
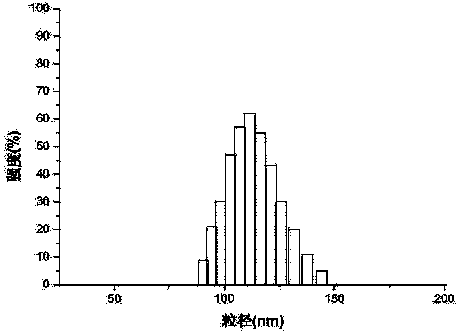
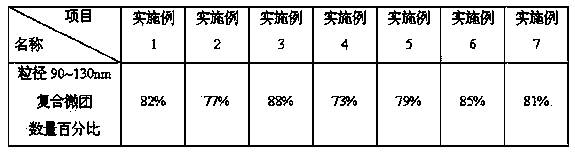
Image
Examples
Embodiment 1
[0041] 1) Mix 1000 mg of hydrogenated soybean lecithin, 200 mg of cholesterol, 100 mg of a compound of chenodeoxycholic acid and sodium taurodeoxycholate at a ratio of 1:1 by weight, and 100 mg of vitamin E to obtain a total mixture, and add the total mixture to 80m1 , with a volume fraction of 99.5% ethanol solution, and ultrasonically dissolve it in a water bath to form an organic solution ①; the organic solution ① uses CO 2 Dissolve in supercritical extraction equipment, the temperature of the extraction kettle is 60°C, the pressure of the extraction kettle is 30MPa, the temperature of the separation kettle is 65°C, the pressure of the separation kettle is 10MPa, and an organic solution is formed②;
[0042] 2) Add 300mg of entecavir into 0.01mol / L, pH7.4 phosphate buffer according to the mass volume ratio of 8g / L, and dissolve it by ultrasonication in a water bath to form entecavir into a solution;
[0043] 3) Inject the organic solution ② into the entecavir solution obtain...
Embodiment 2
[0047] 1) Mix 950 mg of soybean lecithin, 250 mg of cholesterol, 120 mg of a compound of chenodeoxycholic acid and sodium taurodeoxycholate at a ratio of 1:1 by weight and 180 mg of vitamin E to obtain a total mixture, and add the total mixture to 70m1, volume In the ethanol solution with a fraction of 98.0%, the water bath ultrasonically dissolves it to form an organic solution ①; the organic solution ① uses CO 2 Dissolved in supercritical extraction equipment, the temperature of the extraction kettle is 55°C, the pressure of the extraction kettle is 25MPa, the temperature of the separation kettle is 60°C, and the pressure of the separation kettle is 12MPa to form an organic solution②;
[0048] 2) Add 390mg of entecavir into 0.01mol / L, pH7.4~7.6 phosphate buffer according to the mass-volume ratio of 7.5g / L, and dissolve it by ultrasonication in a water bath to form entecavir into a solution;
[0049] 3) Inject the organic solution ② into the entecavir solution obtained in ste...
Embodiment 3
[0053] The present embodiment adopts following preparation method:
[0054] 1) Mix 400mg of soybean lecithin, 100mg of cholesterol, 50mg of the complex of chenodeoxycholic acid and sodium taurodeoxycholate at a weight ratio of 1:1, and 20mg of vitamin E to obtain a total mixture, and add the total mixture to 50m1, In an ethanol solution with a volume fraction of 99.0%, dissolve it in a water bath with ultrasonic waves to form an organic solution ①; use CO 2 Dissolve in supercritical extraction equipment, the temperature of the extraction kettle is 60°C, the pressure of the extraction kettle is 30MPa, the temperature of the separation kettle is 65°C, the pressure of the separation kettle is 10MPa, and an organic solution is formed②;
[0055] 2) Add 100mg of entecavir into 0.01mol / L, pH 7.4~7.6 phosphate buffer according to the mass volume ratio of 6g / L, and dissolve it by ultrasonication in a water bath to form entecavir into a solution;
[0056] 3) Inject the organic solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com