A kind of Zidolamid bisvudine tablet that is easy to dissolve and preparation method thereof
A lamivudine and low-substitution technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve problems such as differences in drug dissolution and bioavailability, and achieve The effect of high dissolution rate, high bioavailability and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
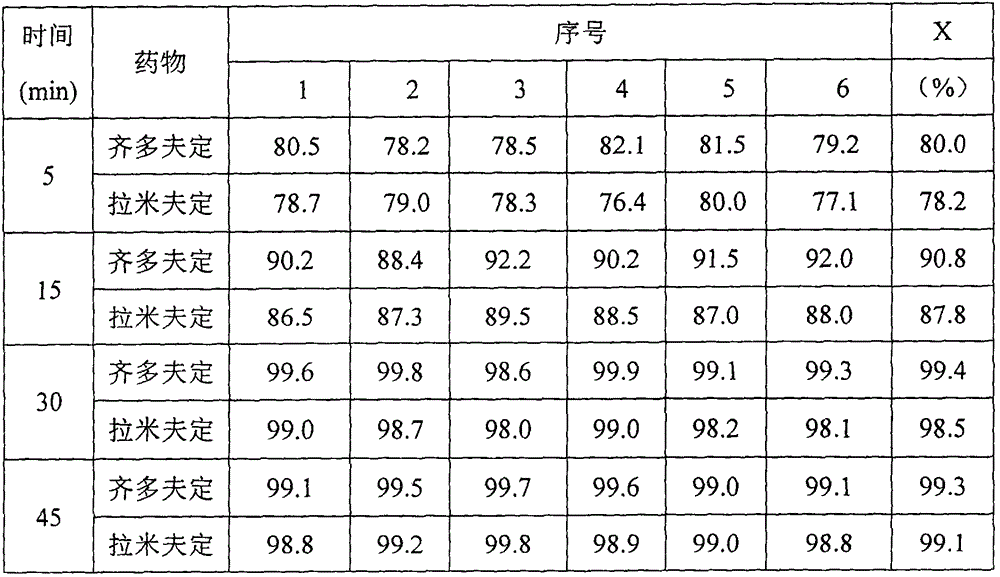
Embodiment 1
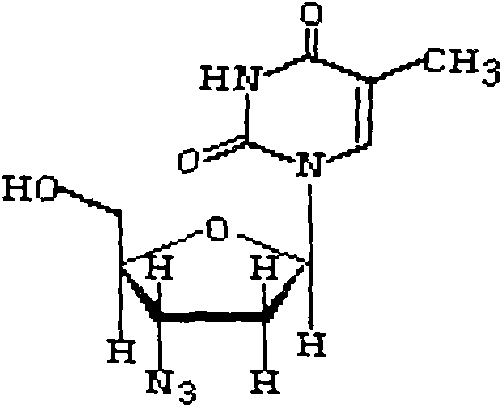
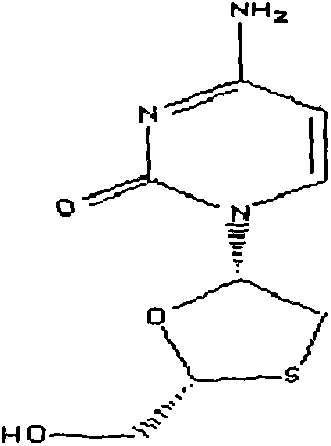
[0029] Weigh 300g of zidovudine, 150g of lamivudine, 40g of lactose, 60g of microcrystalline cellulose, 6g of magnesium stearate, 160g of 3% low-substituted hydroxypropyl cellulose mixed solution
[0030] The preparation method of zidolamidivudine tablet comprises the following processing steps:
[0031] (1) Accurately weigh zidovudine, lamivudine, lactose, microcrystalline cellulose, magnesium stearate and pass through 80 mesh or 100 mesh sieve respectively of above-mentioned weight portion;
[0032] (2) Accurately weigh the low-substituted hydroxypropyl cellulose, add purified water, the mass ratio of the two is 3:97, stir well to make the above-mentioned 3% low-substituted hydroxypropyl cellulose mixed solution by weight;
[0033] (3) mix zidovudine, lamivudine, lactose, microcrystalline cellulose evenly;
[0034] (4) Then add the prepared 3% low-substituted hydroxypropyl cellulose mixture into step (3) to make a soft material and granulate it through a 20-30 mesh screen, ...
Embodiment 2
[0039] Weigh 300g of zidovudine, 150g of lamivudine, 50g of lactose, 50g of microcrystalline cellulose, 6g of magnesium stearate, 170g of 3% low-substituted hydroxypropyl cellulose mixed solution
[0040] All the other are the same as embodiment 1
Embodiment 3
[0042] Weigh 300g of zidovudine, 150g of lamivudine, 60g of lactose, 60g of microcrystalline cellulose, 8g of magnesium stearate, 155g of 3% low-substituted hydroxypropyl cellulose mixed solution
[0043] All the other are the same as embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com