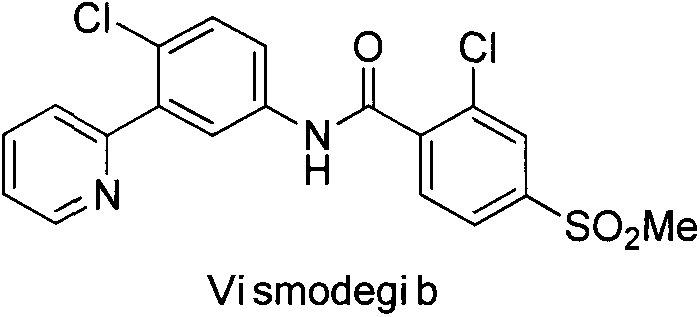
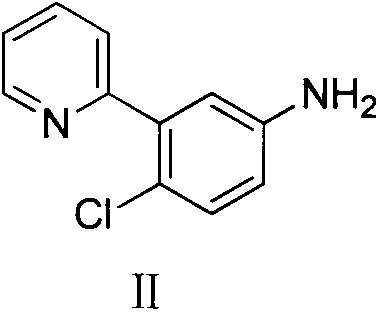
Preparation method for vismodegib
A compound and hydroxyl technology, applied in the field of preparation of 2-chloro-N-phenyl)-4-benzamide and its intermediates, can solve problems such as Hedgehog pathway activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Embodiment 1: Preparation of 1-oxo-2-(3-nitrophenyl)pyridine
[0143]
[0144]Add 30mmol 1-oxopyridine, 10mmol 3-bromo-1-nitrobenzene, 12mmol potassium carbonate, 0.05mmol palladium acetate and 0.15mmol tri-tert-butylphosphine tetrafluoroborate, 40ml toluene, argon to a 100ml three-necked flask After three exchanges, reflux for 2 d under argon protection, then pour the reaction solution into 100 ml of ethyl acetate, filter, wash the filtrate with saturated brine, dry, evaporate the solvent under reduced pressure, and perform column chromatography (mobile phase V / V : dichloromethane / methanol=200 / 1), the fractions were collected and the solvent was evaporated under reduced pressure to obtain a light yellow solid with a yield of 70%.
[0145] 1 HNMR (500Hz, DMSO-d6): 8.79(s, 1H), 8.40(t, 1H), 8.32(q, 1H), 8.24(d, 1H), 7.78(q, 2H), 7.48(m, 2H) ;
[0146] MS: 217.2 ([M+H] + ).
Embodiment 2
[0147] Embodiment 2: Preparation of 1-oxo-2-(3-nitrophenyl)pyridine
[0148]
[0149] Add 30mmol of 1-oxopyridine, 10mmol of 3-bromo-1-nitrobenzene, 12mmol of potassium carbonate, 0.1mmol of cuprous iodide and 0.10mmol of 1,10-phenanthroline to a 100ml three-necked flask, 40ml of xylene, argon After three exchanges, reflux for 2 days under the protection of argon, cool down to room temperature, then pour the reaction system into 100ml of dichloromethane, filter, wash the filtrate with saturated brine, dry, filter, evaporate the filtrate to remove the solvent under reduced pressure, and perform column chromatography (Mobile phase V / V: dichloromethane / methanol=200 / 1), a light yellow solid was obtained with a yield of 46%.
Embodiment 3
[0150] Embodiment 3: the preparation of 2-(3-nitrophenyl) pyridine
[0151]
[0152] Add 3.0mmol of 1-oxo-2-(3-nitrophenyl)pyridine, 15mmol of phosphorus trichloride and 30ml of chloroform into a 100ml eggplant-shaped bottle, heat and reflux for 12h, pour the reaction solution into 100ml of water, and add acetic acid Extracted with ethyl ester (50ml×2), combined the organic phases, dried, evaporated the solvent under reduced pressure, and recrystallized from n-hexane to obtain a light yellow solid with a yield of 92%.
[0153] 1 HNMR (500Hz, CDCl 3 ): 8.86(s, 1H), 8.76(d, 1H), 8.40(d, 1H), 8.27(d, 1H), 7.85(q, 2H), 7.66(t, 1H), 7.36(m, 1H) ;
[0154] MS: 201.1 ([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com