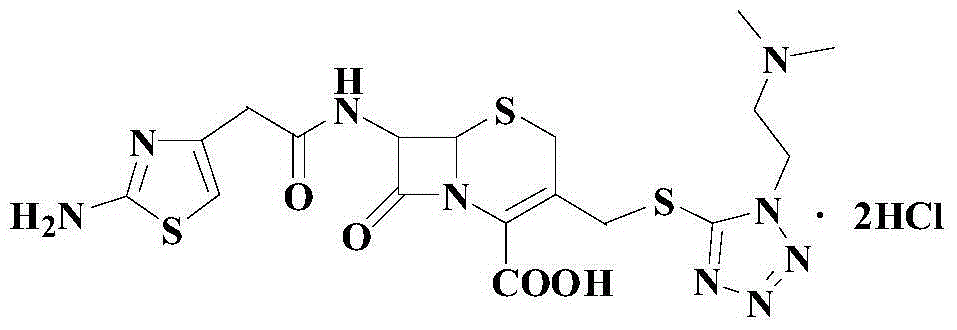
A preparation method of cefotiam hydrochloride
A cefotiam hydrochloride and a combination technology, which is applied in the field of preparation of cefotiam hydrochloride, can solve the problems of low yield and product purity, difficult reaction control, difficult operation, etc., achieve stable yield, reduce the amount of formic acid, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
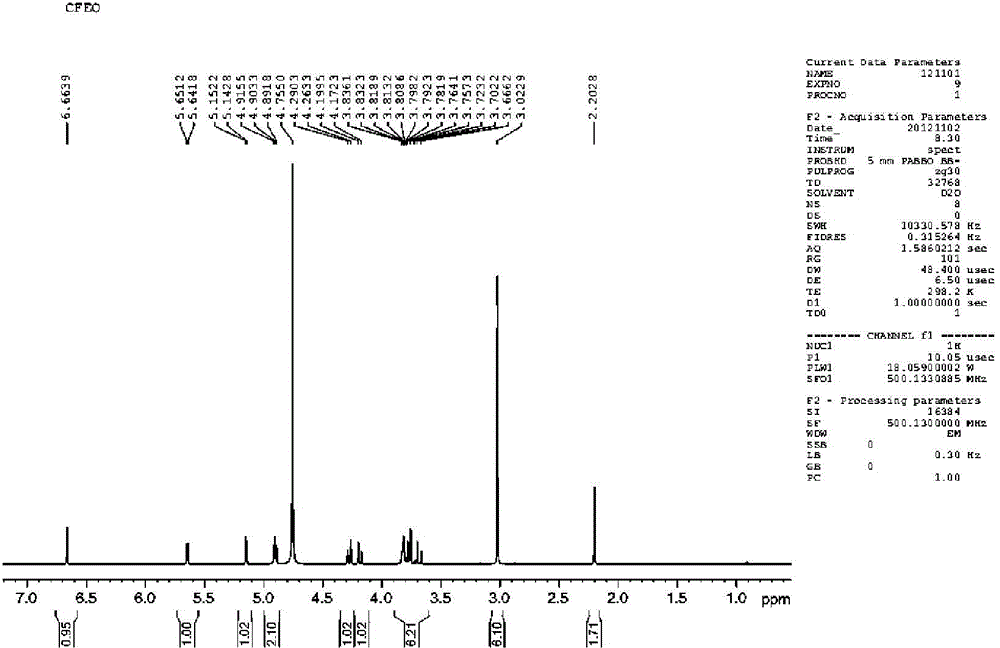
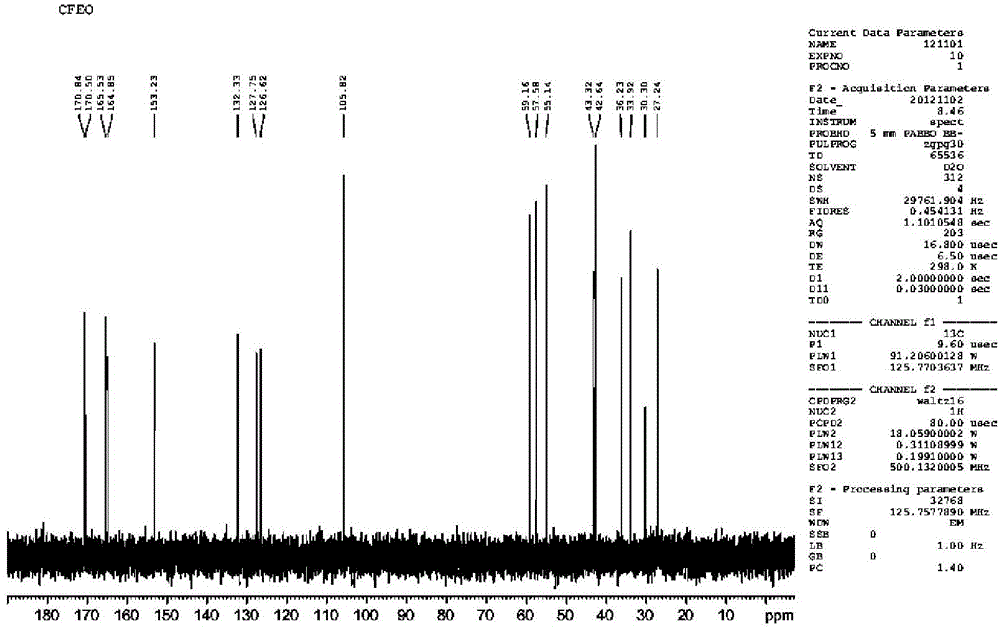
Image
Examples
Embodiment 1
[0041] The synthesis of embodiment 1FATA
[0042] 326.4g (0.8mol) Ac 2 O, 220.8g (4.8mol) HCOOH was added dropwise under stirring, the temperature was raised, and stirred at room temperature for 0.5h, the system was in a colorless and clear state. Add 126.4g (0.8mol) ATA, raise the temperature to 25°C, stir and react at 30-50°C for 4h, and the reaction solution changes from turbid to clear and then turbid again in about 1h. TLC monitored the reaction to be complete. Cool down in an ice-water bath, add 500g of water dropwise, and control the internal temperature to ≤35°C; add ammonia water dropwise to adjust the pH=2.8 to 3.0, control the internal temperature to ≤15°C, share 150.0g of deammonized water, and stir at 0 to 10°C for 0.5h. Suction filtration, washing with tap water (150g×3). -0.08~-0.09MPa, dried at 75°C for 10h, FATA was obtained as off-white solid 115.2g, yield 77.4%, HPLC purity 96.5%.
Embodiment 2
[0043] The synthesis of embodiment 2FATAA
[0044] Suspend 9.3g (0.05mol) of FATA in 93g of acetonitrile, cool down to -10~10°C, white suspension, add 6.5g (0.06mol) of ethyl chloroformate dropwise, stir and react at -10~10°C for 1h, the reaction solution It became slightly clear, and the reaction was monitored by TLC to complete. The acetonitrile solution of FATAA was directly put into the next step reaction, and the HPLC purity was 95.4%.
Embodiment 3F
[0045] The synthesis of embodiment 3FCEFO
[0046]15.7g (0.033mol) of 7-ACMT, 75g of acetonitrile, cooled to -10-10°C under N2 protection, 31.5g (0.17mol) of tri-n-butylamine was added dropwise, the light yellow suspension turned into a brown clear liquid. Add this liquid dropwise to the mixed anhydride prepared in "Example 2", control the internal temperature to ≤ -15°C, and complete the dropwise addition within 0.5h. Stir and react at -40~-20°C for 2h. Reaction system state: near white suspension → powdery white suspension → brownish yellow clear liquid → near white suspension. Suction filtration, washing with cold acetonitrile (20g×2; -20~-15℃), washing with acetone (20g×1). -0.08~-0.09MPa, dried at 35°C for 5h, FCEFO was obtained as off-white solid 16.8g, yield 68.6%, HPLC purity 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com