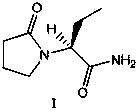
Method for purifying levetiracetam crude product
A purification method and a crude product technology are applied in the field of purification of levetiracetam crude products, can solve the problems of large usage, low yield of levetiracetam, low product purity, etc., achieve cost reduction, less solvent consumption, good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment one: a kind of purification method of levetiracetam crude product
[0025] The purification method consists of the following steps in turn:
[0026] In the first step, the crude product of levetiracetam is added to the crystallization system, and the weight ratio of the crystal system to the crude product of levetiracetam is 1~10:1; wherein, the crystallization system is C3~C6 ketones and A mixed system of purified water, the weight ratio of the C3~C6 ketones to the purified water is 10~50:1;
[0027] In the second step, the system obtained in the first step is heated and dissolved, and the dissolution temperature is 50-90° C.;
[0028] The third step is to filter the system obtained in the second step to obtain a filtrate, and then cool the filtrate to a predetermined temperature for crystallization, and the crystallization temperature is -30~30°C;
[0029] In the fourth step, the system obtained in the third step is filtered, washed, and dried to obtain t...
Embodiment 2
[0030] Embodiment two: a kind of purification method of levetiracetam crude product
[0031] (A) Synthesis of crude levetiracetam
[0032] 84 g of anhydrous sodium sulfate were added to a suspension of 69.25 g (0.5 mol) (S)-2-aminobutanamide hydrochloride in 600 ml of dichloromethane at room temperature. The mixture was cooled to 0°C and 115 g of powdered potassium hydroxide were added, followed by 8.1 g (0.025 mol) of tetrabutylammonium bromide dissolved in 100 ml of methylene chloride. A solution obtained by dissolving 77.5g of 4-chlorobutyryl chloride in 100ml of dichloromethane was added dropwise at 0°C under vigorous stirring. After reacting for 5 hours, 29 g of powdered potassium hydroxide was added. Two hours later, filter the reaction system with diatomaceous earth, evaporate the filtrate under reduced pressure, add 100ml toluene to the residue, heat and reflux for 30 minutes, filter, evaporate the filtrate under reduced pressure to obtain 80g of levetiracetam crude ...
Embodiment 3
[0035] Embodiment three: a kind of purification method of levetiracetam crude product
[0036] Into the reaction flask, add 100g of acetone, 5g of purified water, add 20g of the crude levetiracetam obtained in Example 2, heat to 50°C and stir for 30 minutes, filter, and cool the filtrate to -5°C for crystallization for 5 hours, filter , washed with a small amount of cold acetone, and vacuum-dried at 40°C to obtain 15.3 g of the finished product. The yield of levetiracetam was 76.5%. The purity of the finished product measured by liquid chromatography was 99.98%, and the chirality was 99.95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com