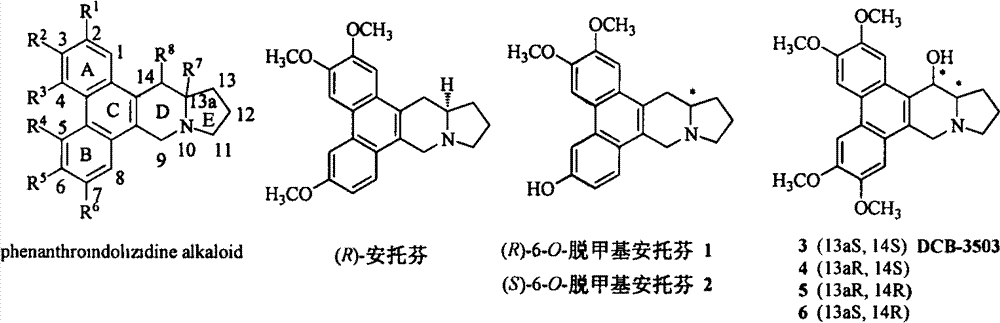
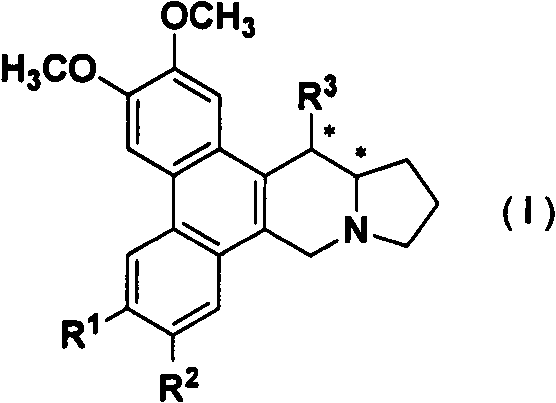
Phenanthroindolizidine alkaloid glycosylation product, 6-site derivatization product, and preparation methods and plant virus resistance activities of phenanthroindolizidine alkaloid glycosylation product and 6-site derivatization product
A technology of phenanthroindolizidine and indolizidine, applied in the field of anti-plant virus activity, can solve the problems that the 6-position substituent has not been studied, and the method of 6-position derivatization is lack of reports.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
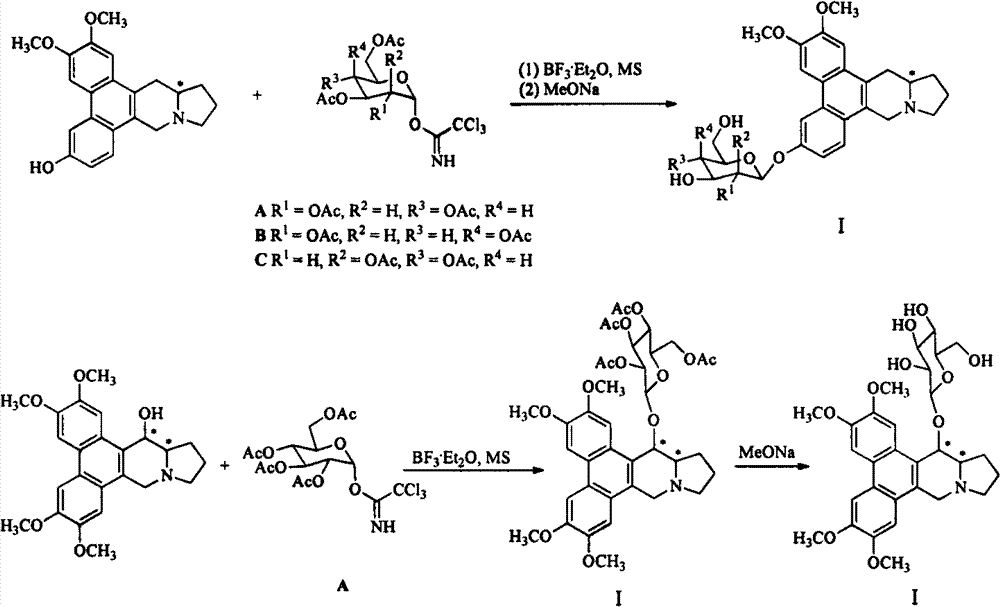
[0033] Example 1: Synthesis of (S)-6-O-desmethylantorphine oxyside I-1~I-4 and 14-hydroxy sylphenoxyside I-5~I-6
[0034] Synthesis of (S)-6-O-desmethylantorfenoxoside I-1~I-4
[0035]
[0036] Add (S)-6-O-desmethylantorphine 2 (0.40g, 1.15mmol) to 40mL of dichloromethane, under the protection of argon, add Molecular sieves, stirred at room temperature for 0.5h. Add 0.2mL BF at low temperature 3 ·Et 2 O, stirred for 0.5h, added dropwise a dichloromethane solution of sugar trichloroacetimidate A (or B, C) (0.75g, 1.20mmol), reacted for 4h, TLC monitored the reaction to be complete, added water to quench the reaction, and separated , extracted the aqueous phase with dichloromethane, dried over anhydrous magnesium sulfate, precipitated, and column chromatographed (ethyl acetate-dichloromethane:methanol=30:1) to obtain fully acetylated glycosides. Add the obtained glycosides to 15 mL of methanol, add sodium methoxide to pH = 9-12, stir at room temperature for 1 h, precipit...
Embodiment 2
[0046] Example 2: A method for glycosylation of (S)-6-O-demethylantorphine and 14-hydroxysilimenine using 1,2,3-triazole as a linking arm.
[0047]
[0048] Synthesis of (13aS)-6-O-propargylantorphine (I-28) Dissolve (S)-6-O-desmethylantorphine 2 (0.81 g, 2.33 mmol) in 15 mL of N,N- In dimethylformamide, add Cs 2 CO 3 (0.91g, 2.79mmol), stirred at room temperature for 0.5h, slowly added dropwise a solution of propargyl bromide (2.79mmol) in 10mL N,N-dimethylformamide, and reacted until the reaction was complete as monitored by TLC. Add 30mL of ethyl acetate and 30mL of water, separate the liquid, extract the aqueous phase with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, precipitate, and perform column chromatography (ethyl acetate as eluent ), the white solid product I-28 (0.77g) can be obtained, the yield is 85%, and the melting point is 195-197°C; 1 H NMR (400MHz, CDCl 3 ): δ8.04(br, 1H), 7.91(s, 1H), ...
Embodiment 3
[0063] Example 3: A method for glycosylation of (R) / (S)-6-O-demethylantorphine and 14-hydroxysilicateline with carbamoyloxy as a linking arm.
[0064]
[0065]The alkaloid (R)-6-O-desmethylantorphine (1) or (S)-6-O-desmethylantorphine (2) (0.18 g, 0.52 mmol) was dissolved in dichloromethane (40mL), add 2-deoxy-2-isocyanato-1,3,4,6-tetra-O-acetyl-β-D-glucose (G) (0.27g, 0.62mmol), add 0.4mL Triethylamine, the reaction was complete as detected by TLC, solvent removal, and normal pressure column chromatography (ethyl acetate—dichloromethane:methanol=10:1) to obtain product I-19 or I-20.
[0066] Dissolve 14-hydroxyalkaloid 3 (or 4,5,6) (0.18g, 0.44mmol) in dichloromethane (40mL), add 2-deoxy-2-isocyanato-1,3,4,6 -Tetra-O-acetyl-β-D-glucose (G) (0.76g, 1.76mmol), add 0.4mL triethylamine, TLC detects that the reaction is complete, precipitation, normal pressure column chromatography (ethyl acetate: petroleum Ether=10:1) to obtain product I-21 (or I-22, I-23, I-24).
[0067] (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com