Application of lunasin polypeptide in medicine for preventing and treating cataract
A cataract and drug technology, applied in the field of biopharmaceuticals, can solve problems such as cataract occurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Preparation of Lunasin Eye Drops
[0015] Lunasin eye drops formula: Lunasin, 0.04g; Sodium hyaluronate, 0.1g; Boric acid, 1.116g; Borax, 0.191g; Sodium chloride, 0.22g; Disodium edetate, 0.5g ; Ethyl p-hydroxybenzoate, 0.03g. Dilute to 100ml with water for injection.
[0016] Lunaxin eye drops preparation process:
[0017] 1. Under the condition of 60-80℃ water bath, stir quickly and slowly add 0.1g of sodium hyaluronate into 70ml of water for injection to make it completely dispersed and fully swell, so as not to make it stick together, stir for about 30 minutes can be completely dissolved.
[0018] 2 Add boric acid, borax, sodium chloride, sodium edetate and ethyl p-hydroxybenzoate to the above sodium hyaluronate solution, stir well to dissolve, and then set the volume to 100ml to obtain pH7.4 Eye drops blank matrix.
[0019] 3. Add lunaxin freeze-dried powder to the blank matrix of the above eye drops, stir well to dissolve, filter and sterilize throu...
Embodiment 2
[0020] Example 2 The protective effect of Lunasin on human lens epithelial cells damaged by D-galactose
[0021] Human lens epithelial cells (Human lens epithelial cell line SRA01 / 04, purchased from Cancer Institute, Chinese Academy of Medical Sciences) were used as research objects, and cell viability was measured by MTT method. The experimental group was divided into normal group (blank control), model group (cells were only treated with 250mM D-galactose), lunacin drug group (with 250mM D-galactose and 1μM, 10μM, 20μM, 40μM, 80μM dew Nasin co-treated the cells). Human lens epithelial cells in the logarithmic growth phase were digested with 0.25% trypsin, prepared into cell suspension with DMEM (Gibco) containing 10% fetal bovine serum (Hangzhou Sijiqing Bioengineering Materials Co., Ltd.), and prepared in a volume of 5×10 3 Cells / well were seeded in a 96-well plate, 200 μl per well. When the cells adhered to the wall and grew to occupy 80% of the bottom area of the well,...
Embodiment 3
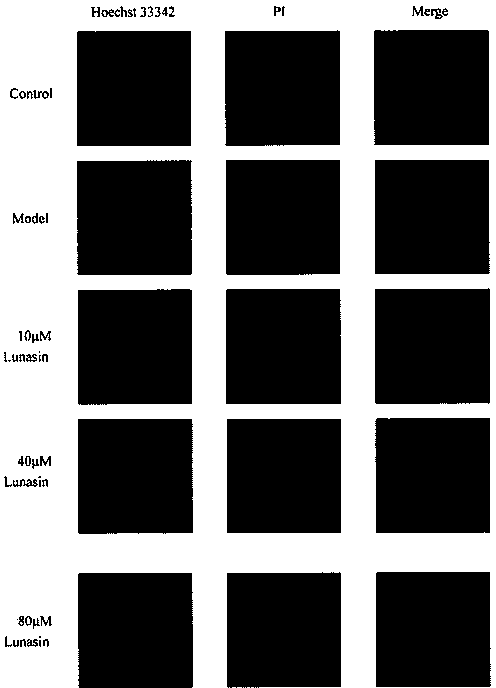
[0025] Example 3 Hoechst33342 / Propidium iodide fluorescence double staining method to observe the inhibitory effect of Lunacin on apoptosis of human lens epithelial cells
[0026]Put the coverslip (18mm×18mm) in the six-well plate in advance. The logarithmic growth phase was digested with 0.25% trypsin, prepared into a cell suspension with DMEM containing 10% fetal bovine serum, and 5×10 6 Cells / well were seeded in a 6-well plate, 2ml per well. When the cells adhered to the wall and grew to occupy 80% of the bottom area of the well, the original medium was discarded, washed once with PBS, and 2ml containing 1% fetal bovine serum was added to each well. DMEM. The normal group was added with 100 μl of PBS as a control; the model group was added with 100 μl of PBS containing D-galactose, and the final concentration of galactose was 250 mM; The final concentration of galactose was 250mM, and the final concentration of lunacin was 10μM, 40μM, and 80μM in turn, at 37°C, 5% CO 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com