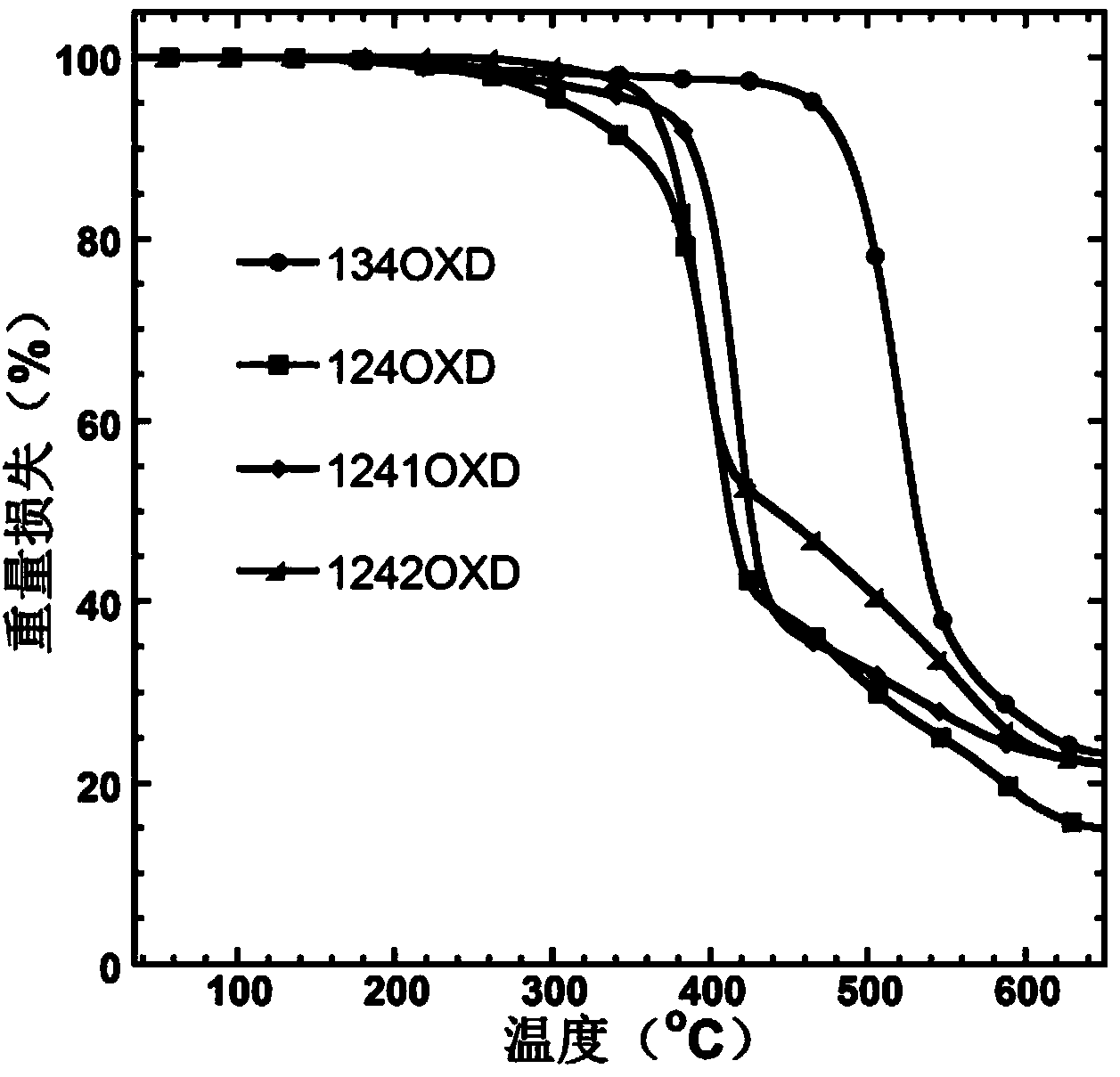
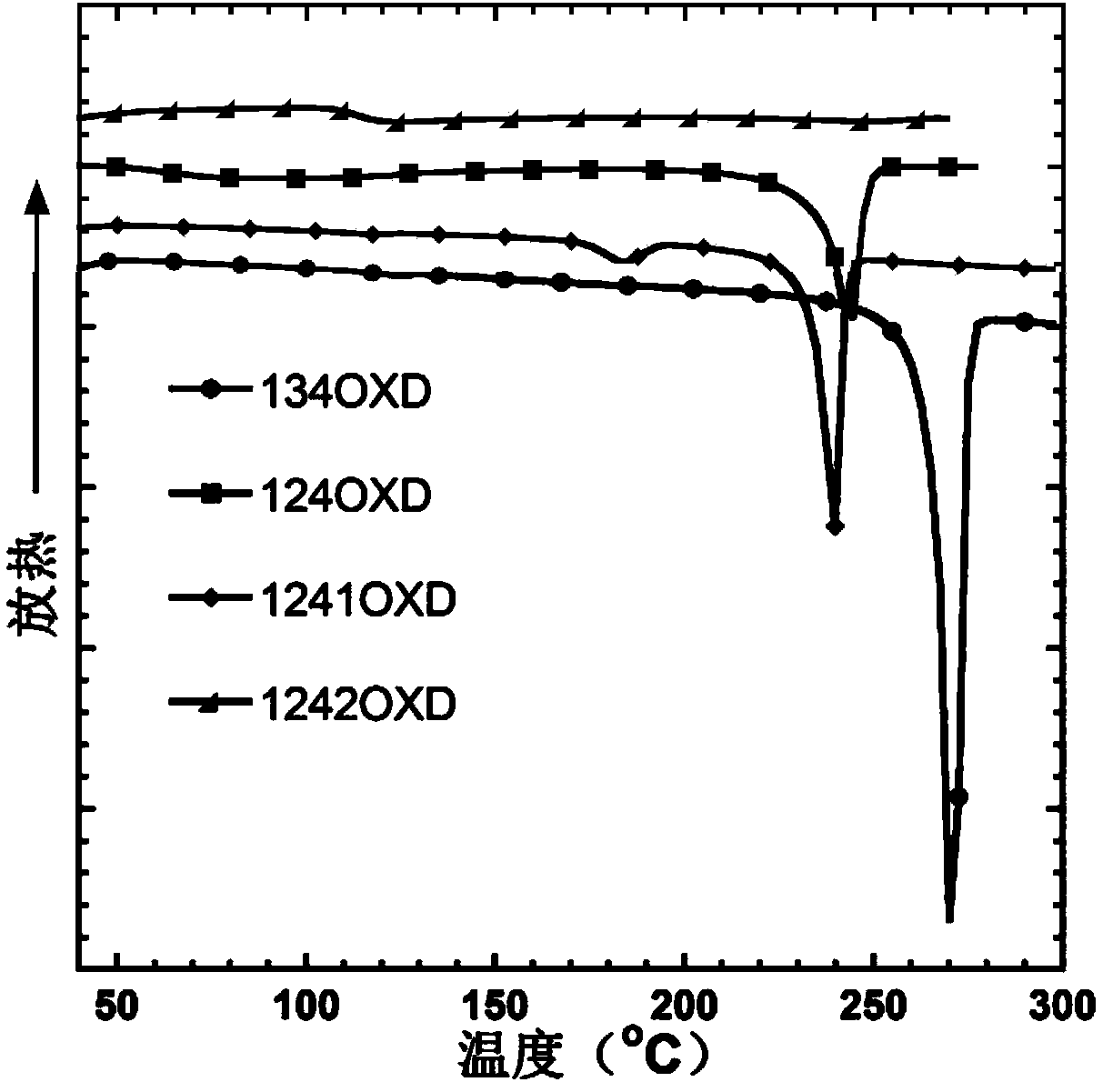
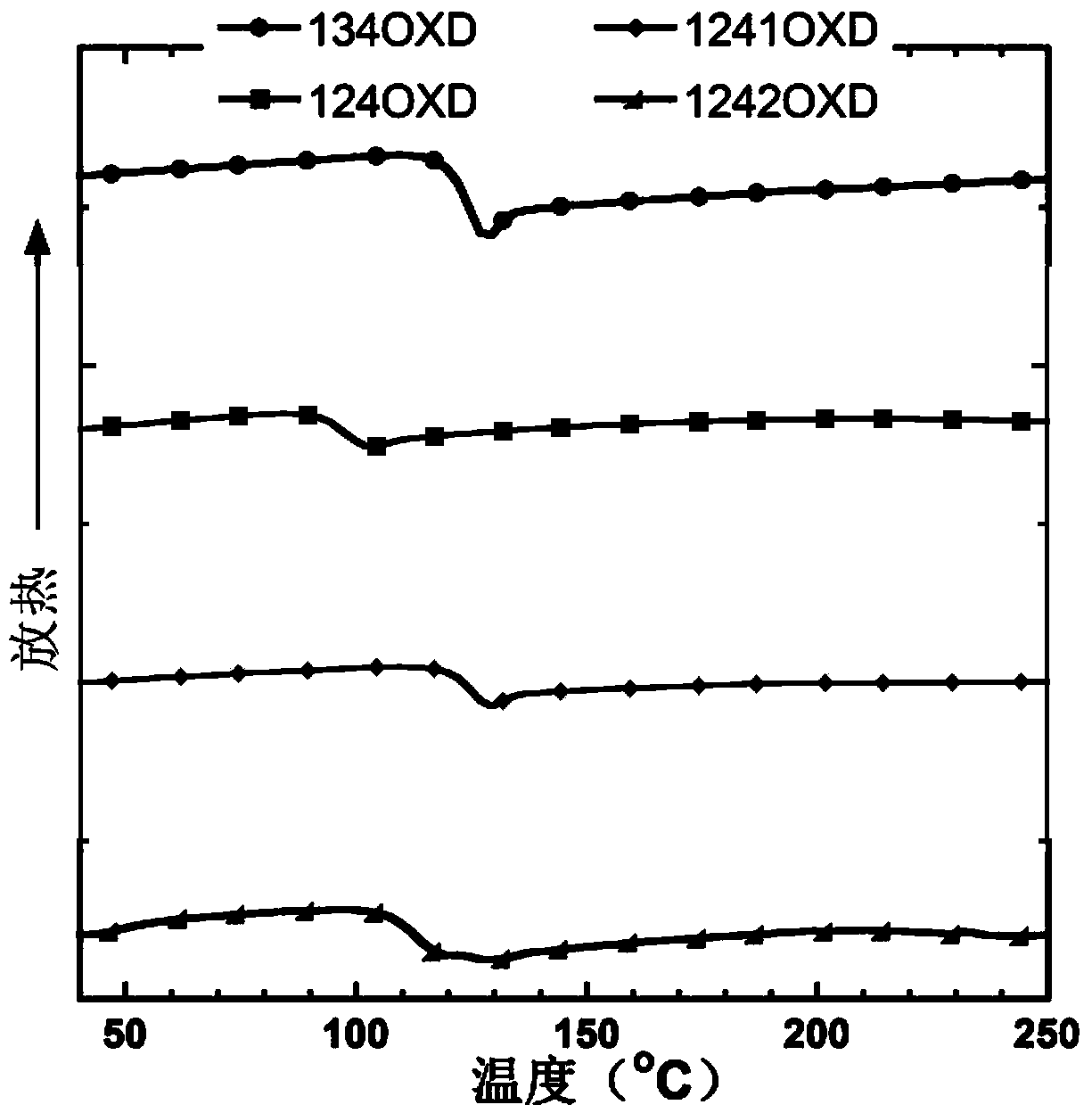
1, 2, 4-oxadiazole micromolecule main body material as well as preparation method and application thereof
A technology of oxadiazole small molecules and host materials, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., and can solve the problems of device performance degradation and annihilation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Step 1: Preparation of 1,3-bis-N-hydroxy-benzamidine (A1)
[0069]
[0070] In an ice bath, sodium carbonate (Na 2 CO 3 ) (8.48g, 80.0mmol) was slowly added to water (27mL) dissolved in hydroxylamine hydrochloride (5.65g, 81.3mmol), and subsequently, the above mixture was poured into a solution of isophthalonitrile (2.56g, 20.0mmol) In absolute ethanol (30mL), reflux for 14h. After cooling to room temperature, the mixture was placed in the refrigerator (2-4° C.) for 12 h. Suction filtration, the obtained solid was washed with water and ethanol, and recrystallized with absolute ethanol to obtain a white solid (2.84g, 14.6mmol). The yield was 73.3%. 1 H NMR (300MHz, MeOD), δ (ppm): 7.92 (m, 1H), 7.71 (d, 1H), 7.68 (d, 1H), 7.42 (t, 1H). 13 C NMR (MeOD, 75MHz), δ (ppm): 153.59, 133.11, 128.22, 127.09, 123.81.Anal.Calcd.for C 8 h 10 N 4 o 2 H, 5.19; N, 28.85. Found: C, 48.84; H, 5.39; N, 28.78.
[0071] Step 2: Preparation of 1,3-bis(5-(3-bromophenyl)-1,2,4-oxa...
Embodiment 2
[0079] Step 1: Preparation of 3-bromo-N-hydroxy-benzamidine (B1)
[0080]
[0081] In an ice bath, sodium carbonate (Na 2 CO 3 ) (5.30g, 50.0mmol) was slowly added into water (14mL) dissolved in hydroxylamine hydrochloride (3.48g, 50.0mmol), and subsequently, the above mixture was poured into 3-bromobenzonitrile (4.56g, 25.0mmol) In anhydrous ethanol (25mL), the reaction was refluxed for 14h. After cooling to room temperature, the mixture was placed in the refrigerator (2-4° C.) for 12 h. Suction filtration, the obtained solid was washed with water and ethanol, and recrystallized with absolute ethanol to obtain a white solid (5.03g, 23.4mmol). The yield was 93.5%. 1 H NMR (300MHz, MeOD), δ (ppm): 7.82 (t, 1H), 7.60 (m, 2H), 7.31 (t, 1H). 13 CNMR(MeOD,75MHz),δ(ppm):152.55,136.00,132.17,129.83,128.82,124.58,121.91.
[0082] Step 2: Preparation of 1,3-bis(3-(3-bromophenyl)-1,2,4-oxadiazol-5-yl)benzene (B2)
[0083]
[0084] Under a nitrogen atmosphere, isophthaloyl c...
Embodiment 3
[0089] Step 1: Preparation of 3,5-bis-(3-bromophenyl)-1,2,4-oxadiazole (C1)
[0090]
[0091] Under a nitrogen atmosphere, add 3-bromobenzoyl chloride (3.0ml, 22.8mmol) to 53ml of anhydrous pyridine dissolved in 3-bromo-N-hydroxy-benzamidine (4.35g, 20.2mmol) with a syringe, It was stirred for 2 days under the condition of an oil bath at 120°C. After cooling to room temperature, the mixture was poured into 300 mL of deionized water. Suction filtration, the obtained solid was washed with water and ethanol, and recrystallized with absolute ethanol to obtain a white solid (5.43g, 14.3mmol). The yield was 70.7%. 1 H NMR (300MHz, CDCl 3 ),δ(ppm):8.38(s,1H),8.33(s,1H),8.12(dd,2H),7.75(d,1H),7.67(d,1H),7.47-7.37(m,2H) . 13 C NMR (CDCl 3 C 14 h 8 N 2 OBr 2 380.3, APCI + -MS(m / z):380.9(M + ).
[0092] Step 2: Preparation of 3,5-bis(3'-(9H-carbazol-9-yl)biphenyl-3-yl)-1,2,4-oxadiazole (124OXD)
[0093]
[0094] Under argon atmosphere, add C1 (0.95g, 2.5mmol), 9-(3-(4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com