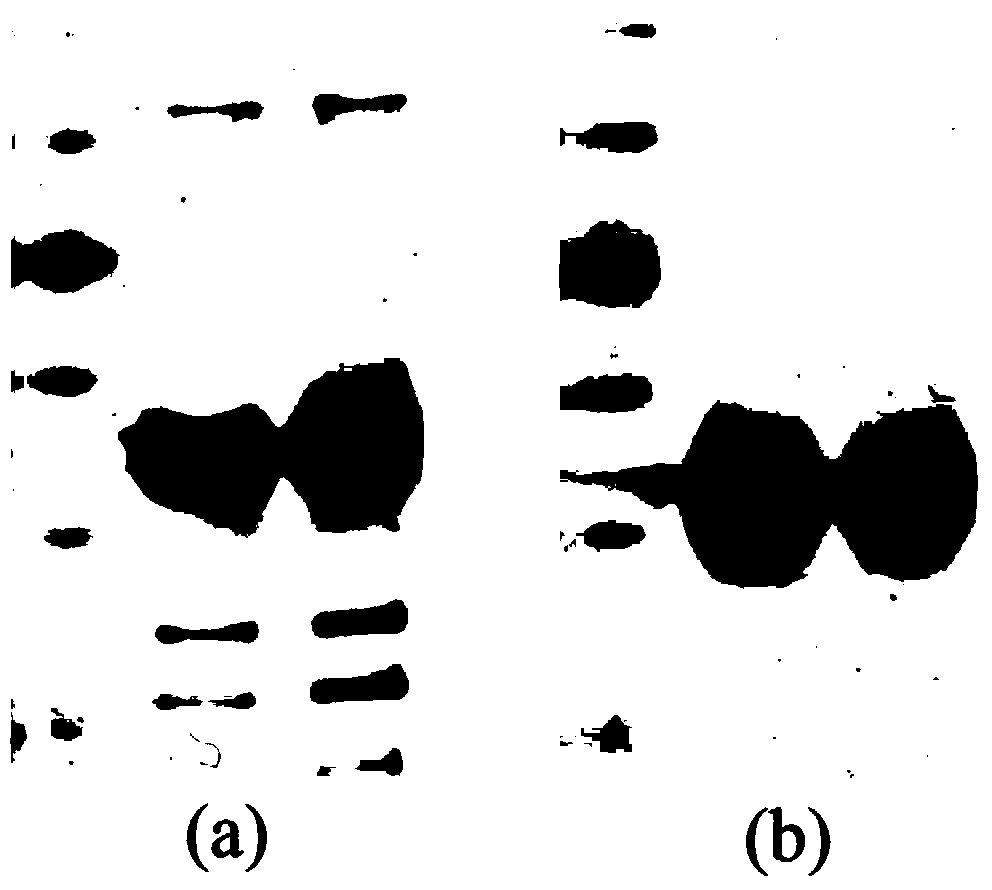Rice Rubisco large-subunit antigen epitope, large-subunit antibody and applications of antibody
An antigenic epitope and rice technology, applied in the fields of molecular biology and immunology, can solve the problem that antibody specificity cannot be guaranteed, and achieve the effect of strong affinity, good specificity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Prediction of Candidate Antigen Epitopes and Artificial Synthetic Antigens
[0024] The ID of the amino acid sequence corresponding to the rice rbcL used in GenBank is P0C510, and the full-length sequence is shown in SEQ ID NO: 3. After analyzing the hydrophilicity, exposure and flexibility of the amino acid sequence, it was selected at the N-terminal of the protein An amino acid sequence is KLTYYTPEYETKDTD (as shown in SEQ ID NO: 1, the sequence is located at positions 21-35 of SEQ ID NO: 3 in the sequence table). After Blast comparison with the rice protein database, it was confirmed that this polypeptide can uniquely identify rbcL.
[0025] In order to couple this sequence with a carrier protein, a cysteine residue is added at the C-terminal of this sequence, so the amino acid sequence synthesized is KLTYYTPEYETKDTDC (as shown in SEQ ID NO: 2), provided by Baiyixin Biotechnology Co., Ltd. The company conducts solid-phase synthesis, and couples succinimi...
Embodiment 2
[0026] Example 2: Serum preparation and purification of polyclonal antibody anti-rbcL
[0027] Animals were immunized with the above-mentioned synthetic antigens, and healthy New Zealand white rabbits aged 3 months and weighing 2 kg were selected for the immunized animals. The specific steps are as follows:
[0028]Firstly, the rabbits were induced before immunization, and 0.5 mL of Freund's complete adjuvant was subcutaneously injected into the limbs, underarms and back to stimulate the local immune response, and immunization was carried out 1 week later. Before the first immunization, blood was taken from the ear vein as a negative control. Antigen (calculated as carrier protein) was washed with PBS buffer (137mM NaCl, 2.7mM KCl, 10mM NaCl 2 HPO 4 , 2mM KH 2 PO 4 , pH7.4) diluted to 1mg·mL -1 . Take 500 μL antigen diluent, add 300 μL PBS buffer and 800 μL Freund’s complete adjuvant (first immunization) or Freund’s incomplete adjuvant (2-4 immunization), mix and emulsif...
Embodiment 3
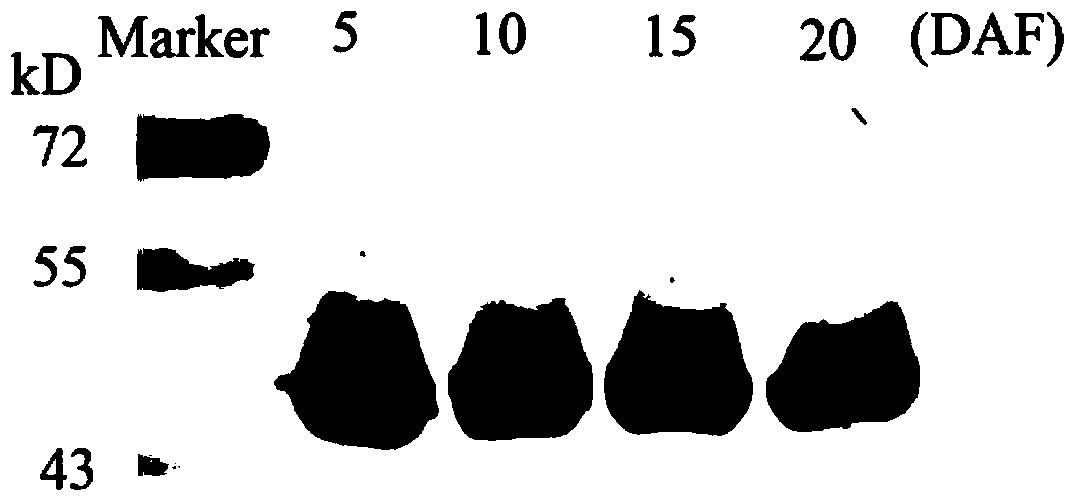
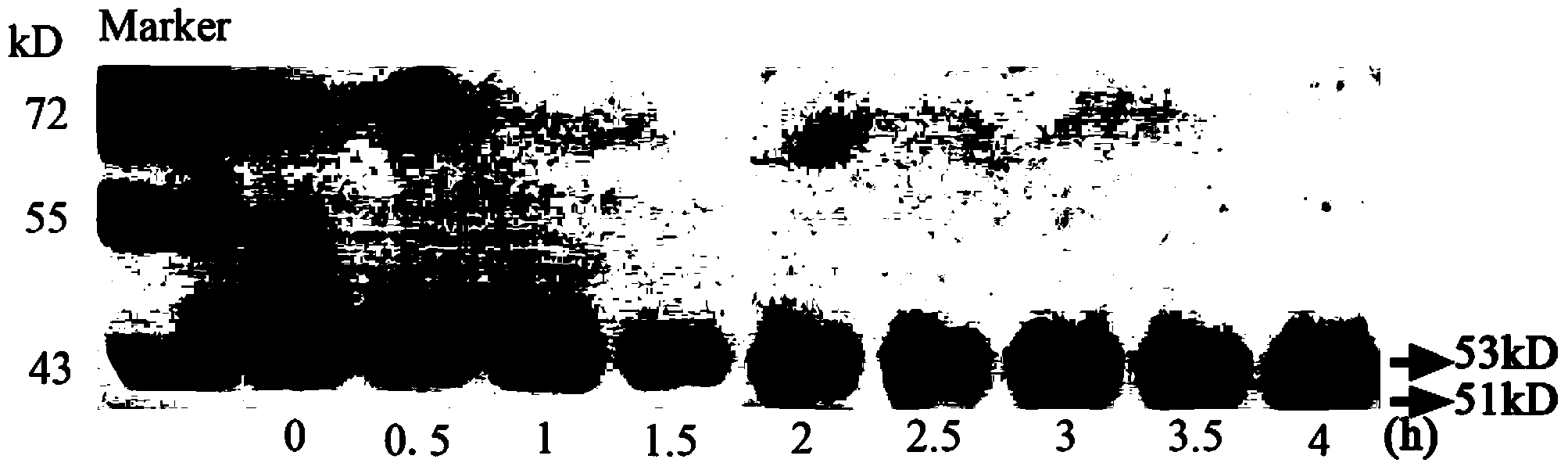
[0032] Embodiment 3: the specificity test of polyclonal antibody anti-rbcL
[0033] 0.1 g of rice leaves were thoroughly ground with liquid nitrogen, and 0.5 mL of pre-cooled protein extraction buffer (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 15 mM β-mercaptoethanol, 1 % [w / v] PVP) for 30 minutes. Centrifuge at 15000g for 30min at 4°C, and the supernatant is Rubisco crude enzyme solution. The soluble protein in Rubisco crude enzyme solution was quantified by Bradford method and stored at -80°C.
[0034] Mix equal volumes of Rubisco crude enzyme solution (2.5 μg protein) and loading buffer for SDS-PAGE electrophoresis separation. The PAGE glue was quickly transferred (high electric field strength) to the nitrocellulose membrane through the tank transfer system, and after the transfer was completed, the blocking solution (20mM Tris-HCl, pH7. Block the nitrocellulose membrane at 37°C for 1h. Using the polyclonal antibody anti-rbcL prepared in Example 2, diluted with blocking soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com