Preparation method and application of graphene-supported non-noble metal electrocatalyst
A non-precious metal, electrocatalyst technology, applied in the direction of metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of performance gap and large particle size of electrocatalysts, etc. Achieve the effect of small size, high density and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
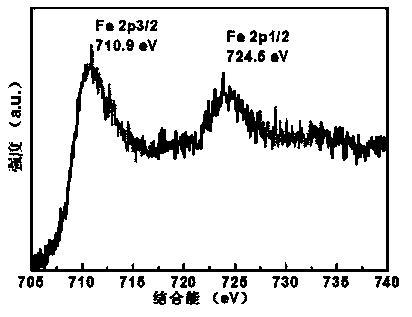
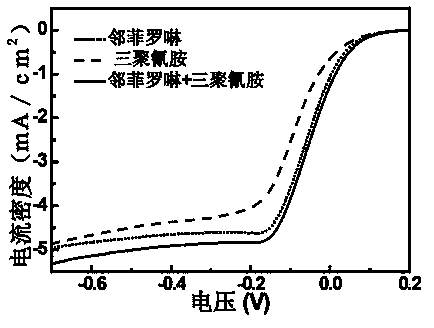
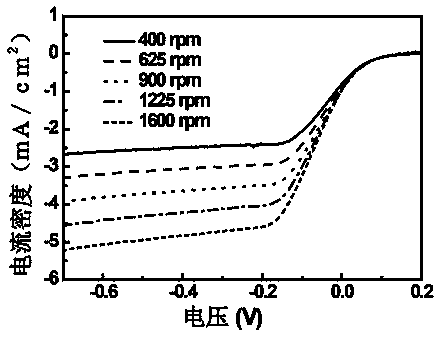
[0040] Place 105mg of o-phenanthroline and 66mg of melamine in 100mL of deionized water, heat in a water bath at 80°C until dissolved, and dissolve 31mg of CoCl 2 ·H 2 O and 66 mg FeCl 3 Dissolve in 10 mL of deionized water respectively, and preheat in a water bath at 80°C after mixing, mix the above two preheated solutions, and reflux and stir in a water bath at 80°C for 30 minutes. Graphene oxide with a mass of 200 mg was weighed in 100 mL of deionized water for 40 min, dispersed evenly, placed in a water bath at 80 ° C for preheating, then added to the above reaction solution, refluxed and stirred for 30 min and then cooled to room temperature. Weigh 400mg of NaBH 4 Dissolve in 50mL deionized water, add dropwise to the reaction system, react for 30min, filter with suction, and dry under vacuum at 65°C. Under the protection of argon atmosphere, the temperature was programmed to 800 °C, stabilized for 2 h, and then naturally cooled to room temperature. 0.5M H 2 SO 4 The...
Embodiment 2
[0055] Different heat treatment temperatures: 105mg of o-phenanthroline and 66mg of melamine were placed in 100mL of deionized water, heated in a water bath at 80°C until dissolved, and 31mg of CoCl 2 ·H 2 O and 66 mg FeCl 3 Dissolve in 10mL deionized water respectively, and preheat in a water bath at 80°C after mixing, mix the above two preheated solutions, and reflux and stir in a water bath at 80°C for 30min. Graphene oxide with a mass of 200 mg was weighed in 100 mL of deionized water for 40 min, dispersed evenly, placed in a water bath at 80 ° C for preheating, then added to the above reaction solution, refluxed and stirred for 30 min and then cooled to room temperature. Weigh 400mg of NaBH 4 Dissolve in 50mL deionized water, add dropwise to the reaction system, react for 30min, filter with suction, and dry under vacuum at 65°C. Under the protection of argon atmosphere, the temperature was programmed to 450°C or 900°C, stabilized for 2 hours, and then naturally cooled ...
Embodiment 3
[0058] Three kinds of nitrogen-containing ligands: 35 mg of phenanthroline, 22 mg of melamine and 27 mg of 4,4’-bipyridine were placed in 100 mL of deionized water and heated in a water bath at 80 °C until dissolved, and 31 mg of CoCl 2 ·H 2 O and 66 mg FeCl 3 Dissolve in 10mL deionized water respectively, preheat in a water bath at 80°C after mixing, mix the above two preheated solutions, reflux and stir in a water bath at 80°C for 30min. Weigh 200 mg of graphene oxide in 100 mL of deionized water for 40 min, disperse evenly, place it in a water bath at 80 ° C for preheating, then add it to the above reaction solution, reflux and stir for 30 min, then cool to room temperature. Weigh 400mg of NaBH 4 Dissolve in 50mL deionized water, add dropwise to the reaction system, react for 30min, filter with suction, and dry under vacuum at 65°C. Under the protection of argon atmosphere, the temperature was programmed to 800 °C, stabilized for 2 h, and then naturally cooled to room te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com