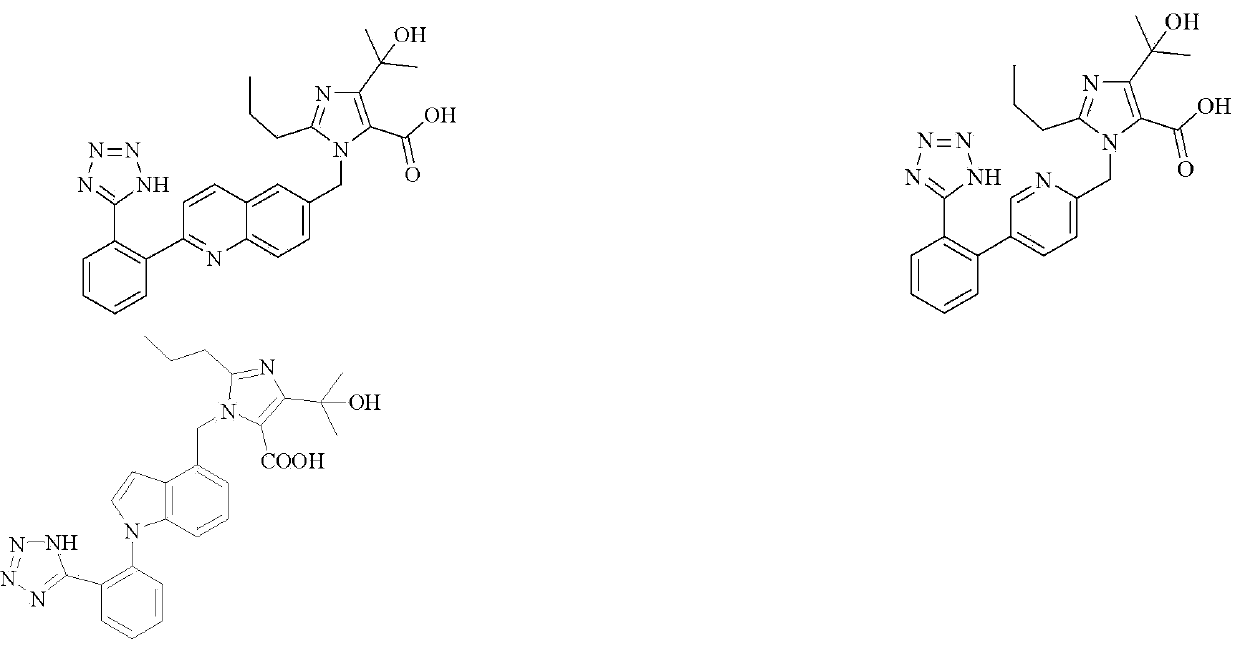
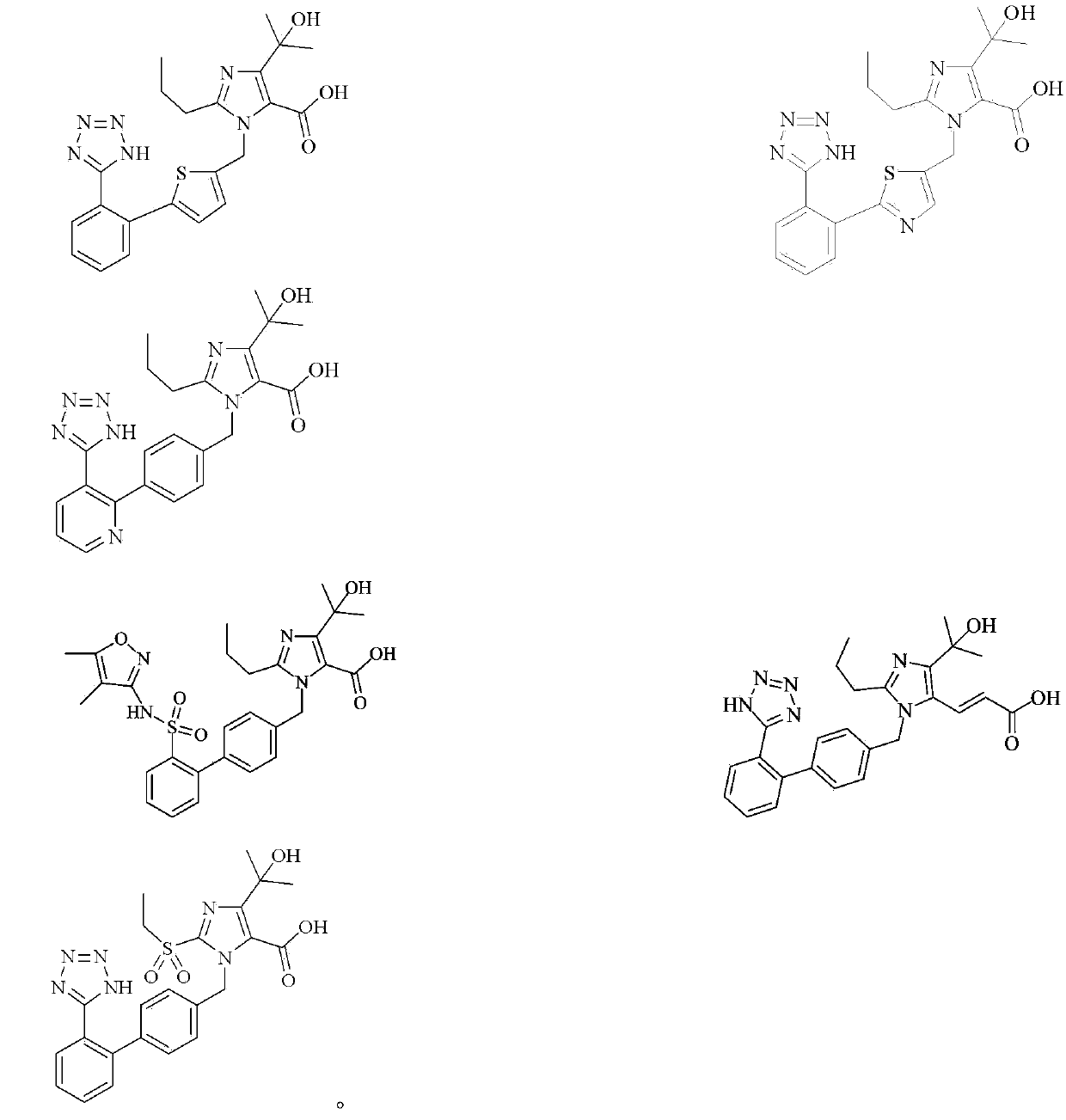
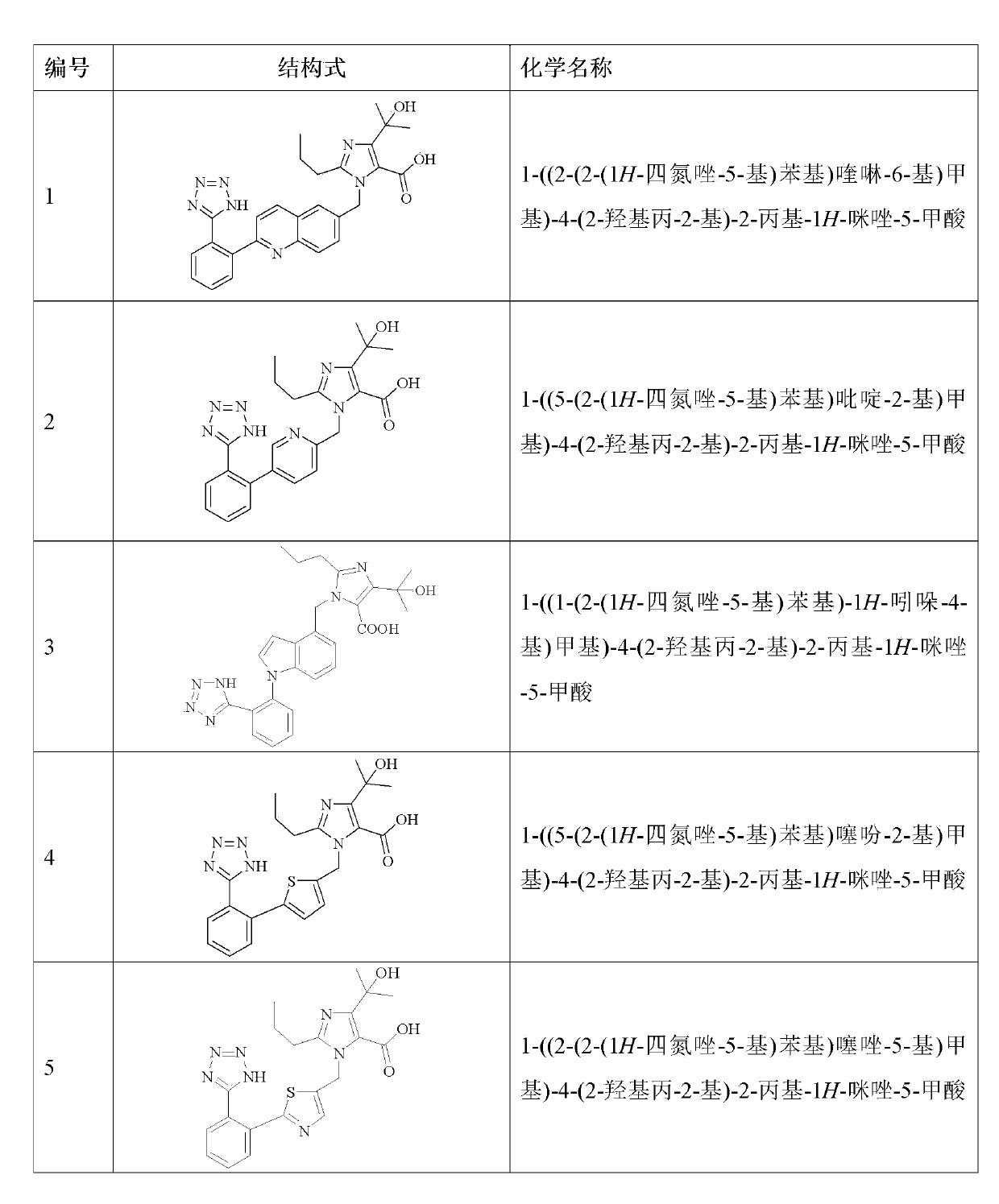
Imidazole carboxylic acid derivative
A carboxyl group, compound technology, applied in the field of medicine, can solve the problems of drug selectivity, unsatisfactory safety, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Example 1: 1-((2-(2-(1H-tetrazol-5-yl)phenyl)quinolin-6-yl)methyl)-4-(2-hydroxypropan-2-yl) - Preparation of 2-propyl-1H-imidazole-5-carboxylic acid (compound 1)
[0152] 1) Preparation of 2-bromo-6-bromomethylquinoline
[0153]
[0154] 2-Bromo-6-methylquinoline (0.13 g, 0.585 mmol), peroxybenzoic acid (0.114 g, 0.643 mmol) and N-bromosuccinimide (0.2 g, 1.12 mmol) were dissolved in 50 mL In carbon chloride, heated to 70°C and stirred for 5 hours, cooled, and the mixture obtained by distillation under reduced pressure was directly used in the next reaction.
[0155] 2) Preparation of ethyl 1-((2-bromoquinolin-6-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1H-imidazole-5-carboxylate
[0156]
[0157] 4-(2-Hydroxypropan-2-yl)-2-propyl-1H-imidazole-5-carboxylic acid ethyl ester (0.287g, 1.2mmol) and 0.165g potassium carbonate were dissolved in 20mL DMF and added while stirring The 2-bromo-6-bromomethylquinoline mixture obtained in the previous step was stirred fo...
Embodiment 2
[0165] Example 2: 1-((5-(2-(1H-tetrazol-5-yl)phenyl)pyridin-2-yl)methyl)-4-(2-hydroxypropan-2-yl)- Preparation of 2-propyl-1H-imidazole-5-carboxylic acid (compound 2)
[0166] 1) Preparation of ethyl 1-((5-bromopyridin-2-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1H-imidazole-5-carboxylate
[0167]
[0168] 4-(2-Hydroxypropan-2-yl)-2-propyl-1H-imidazole-5-carboxylic acid ethyl ester (4g, 16.7mmol), 2-bromomethyl-5-bromopyridine (4.48g, 18mmol ) and potassium carbonate (9, 49mmol) were dissolved in 25mL DMA, stirred at room temperature for 18 hours, diluted with water, extracted with ethyl acetate, combined organic phases, washed with saturated sodium chloride solution, silica gel column (petroleum ether / acetic acid=ethyl ether) Ester 4:1) to give ethyl 1-((5-bromopyridin-2-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1H-imidazole-5-carboxylate Esters 4.2g.
[0169] 2) 1-((5-(2-(1H-tetrazol-5-yl)phenyl)pyridin-2-yl)methyl)-4-(2-hydroxypropan-2-yl)-2- Preparation of eth...
Embodiment 3
[0176] Example 3: 1-((1-(2-(1H-tetrazol-5-yl)phenyl)-1H-indol-4-yl)methyl)-4-(2-hydroxypropan-2 Preparation of -yl)-2-propyl-1H-imidazole-5-carboxylic acid (compound 3)
[0177] 1) Preparation of 2-(4-(hydroxymethyl)-1H-indol-1-yl)benzocyanide
[0178]
[0179] 2-iodobenzonitrile (5g, 4.4mmol), 4-hydroxymethylindole (0.6g, 4mmol), cuprous iodide (76mg, 0.4mmol) and potassium carbonate (0.83g) were placed in 20mL DMSO , heated to 90°C, kept for 12 hours, cooled after completion, poured into water, extracted with ethyl acetate, the combined organic phase was washed with water, saturated sodium chloride, dried, concentrated, silica gel column chromatography (petroleum ether / acetic acid Ethyl ester=10:1), separated to obtain 0.78g of 2-(4-(hydroxymethyl)-1H-indol-1-yl)benzocyanide.
[0180] 2) Preparation of 2-(4-(bromomethyl)-1H-indol-1-yl)benzocyanide
[0181]
[0182] Dissolve 2-(4-(hydroxymethyl)-1H-indol-1-yl)benzocyanide (1g, 4mmol) in 10mL of dichloromethane, cool ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com