Method for adsorbing and separating boron isotope by utilizing modified MCM-41 molecular sieve
A MCM-41, adsorption separation technology, applied in separation methods, separation of different isotopic elements, separation of dispersed particles, etc., can solve the problems of high price, unsuitable for large-scale industrial application, etc., and achieve extremely easy acquisition and high stability , good selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
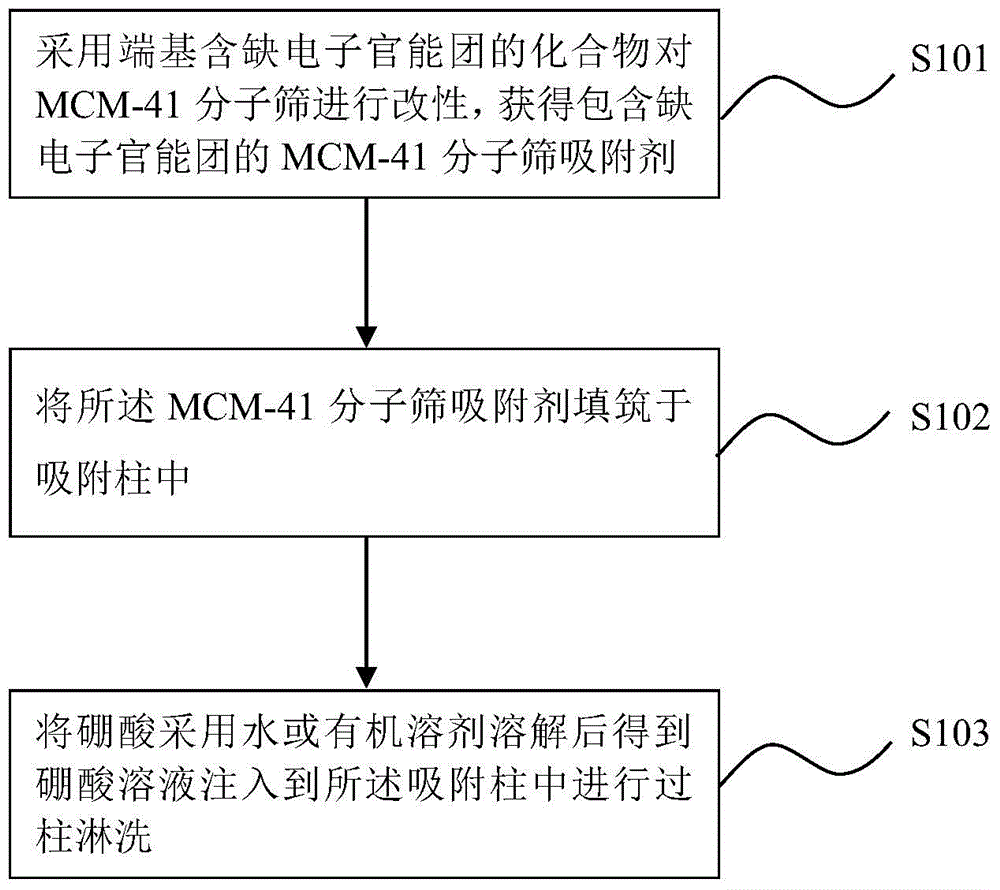
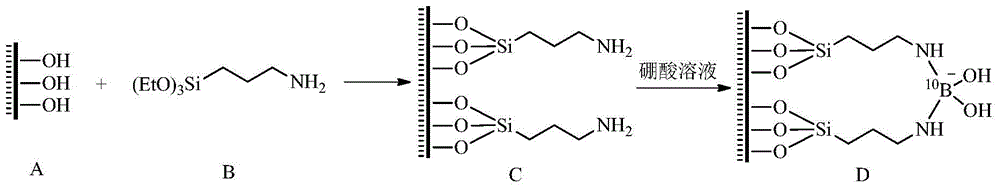
[0036] Example 1 Adsorption and separation of boron isotopes using aminopropyl-modified MCM-41 molecular sieves
[0037] First, add 2.508g CTAB (cetyl ammonium bromide) to 48mL water to obtain a clear and transparent CTAB solution, then add a mixture of 48mL absolute ethanol and 15mL ammonia water to the CTAB solution, continue stirring for 15min, and then add Slowly add 4.7g TEOS (tetraethyl orthosilicate), and continue to stir for 3h until a white solid is formed, filter with suction, wash with 100mL distilled water and 100mL methanol for 2-3 times, and dry the filter cake in an oven at 105°C for 12h , and then calcined at 550°C for 5h, and cooled slowly to obtain white powder solid MCM-41.
[0038] Next, mix MCM-41 and triaminopropyltriethoxysilane at a mass ratio of 2:1, under the protection of nitrogen, use toluene as a solvent, and carry out reflux stirring in an oil bath; the temperature of the oil bath is 70°C , the time is 24h. The solution after the oil bath was co...
Embodiment 2
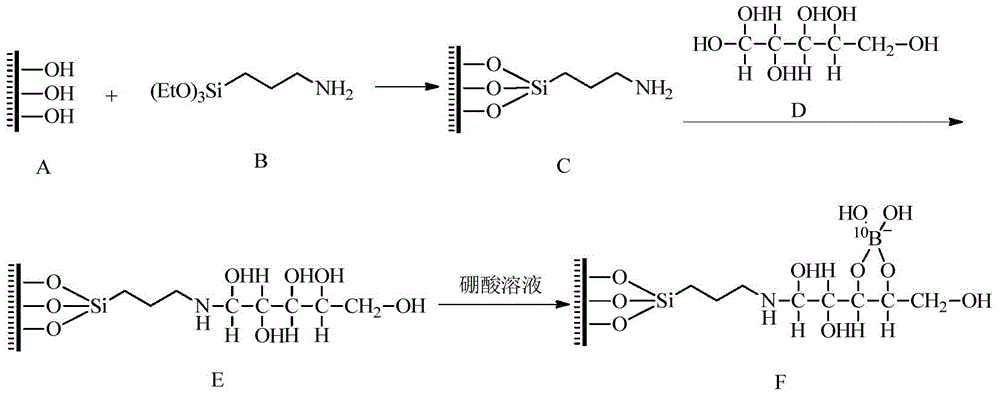
[0044] Example 2 Adsorption and separation of boron isotopes using N-methylglucose-modified MCM-41 molecular sieves
[0045] In this example, after the aminopropyl-modified MCM-41 molecular sieve was prepared with reference to Example 1, the aminopropyl-modified MCM-41 molecular sieve was further mixed and reacted with glucose in an equimolar ratio, and after cooling, Filter, wash and dry to obtain N-methylglucose modified MCM-41 molecular sieve.
[0046] The MCM-41 molecular sieve adsorbent modified by N-methylglucose is filled in an adsorption column with a diameter of 5 cm and a column length of 100 cm, and high-purity boric acid is dissolved in dichloromethane to obtain a boric acid solution with a concentration of 0.7 mol / L. Inject into the adsorption column and perform column elution. The temperature of column elution is 50°C, the adsorption time is 100h, and the flow rate of boric acid solution is controlled at about 3mL / min. If necessary, pressurized injection can be u...
Embodiment 3
[0051] Example 3 Adsorption and separation of boron isotopes using Schiff base-modified MCM-41 molecular sieves
[0052] In this example, after the aminopropyl-modified MCM-41 molecular sieve was prepared in reference to Example 1, the aminopropyl-modified MCM-41 molecular sieve was further mixed with the Schiff base in an equimolar mass ratio, and the 2 Add absolute ethanol under gas protection, add a small amount of glacial acetic acid dropwise, and carry out reflux and stirring in the oil bath; the temperature of the oil bath is 80°C, and the time is 24h. The solution after the oil bath was cooled to room temperature, filtered, and the filtered product was washed with ether-dichloromethane and dried to obtain an off-white powdery solid, that is, Schiff base-modified MCM-41 molecular sieve.
[0053] Fill the MCM-41 molecular sieve adsorbent modified by Schiff base in an adsorption column with a diameter of 10 cm and a column length of 150 cm, and use tetrahydrofuran to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com