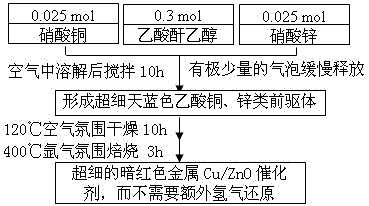
Preparation method of high-activity and free-reduction Cu/ZnO catalyst
A catalyst and high-activity technology, applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems affecting the grain size of metal catalysts, the growth of metal Cu grains, and the remaining amorphous carbon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Table 1 shows the characterization results and low-temperature methanol synthesis reaction data of the Cu / ZnO catalysts prepared by the citric acid-sol-gel method, the formic acid solid-phase grinding method and the acetic anhydride-alcoholic solution method of the present invention. As shown in Table 1, the specific surface area of metal Cu / ZnO catalysts prepared by three methods and the use of N 2 The surface area of metallic copper determined by O chemisorption was citric acid: BET, 27.7 m 2 / g,S Cu ,3.2 m 2 / g; formic acid: BET, 15.29 m 2 / g,S Cu ,4.42 m 2 / g; Acetic anhydride: BET, 41.54 m 2 / g,S Cu ,13.26 m 2 / g. The XRD characteristic peaks of the three prepared catalysts were all attributed to metal Cu and ZnO without any impurity peaks. The grain size of metal Cu and ZnO is calculated using the Scherrer formula, D = Kλ / (β cos θ). Among them, D represents the calculated grain size, K is the Scherrer constant 0.89, λ is the X-ray wavelength of coppe...
Embodiment 2
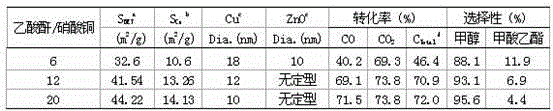
[0032] Example 2. Table 2 shows the characterization, reactivity and formazan selectivity of Cu / ZnO catalysts prepared by different molar ratios of acetic anhydride / copper nitrate. The characterization instruments and conditions are consistent with those in Example 1, and the catalyst evaluation conditions are still synchronized with those in Example 1. From Table 2, we can clearly see that with the increase of acetic anhydride content in the dissolution process, the smaller the crystal grains of Cu in the final prepared catalyst, the higher the reactivity and the higher the methanol selectivity. However, as the amount of acetic anhydride increases, the time required for the solution reduction process is longer, and the maximum reaction time can reach 50 hours before the reduction process can be completed. Because in the oxidation reaction process, with the increase of reducing agent dosage, the reduction process is slower.
[0033] Table 2 Characterization, reactivity and me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com