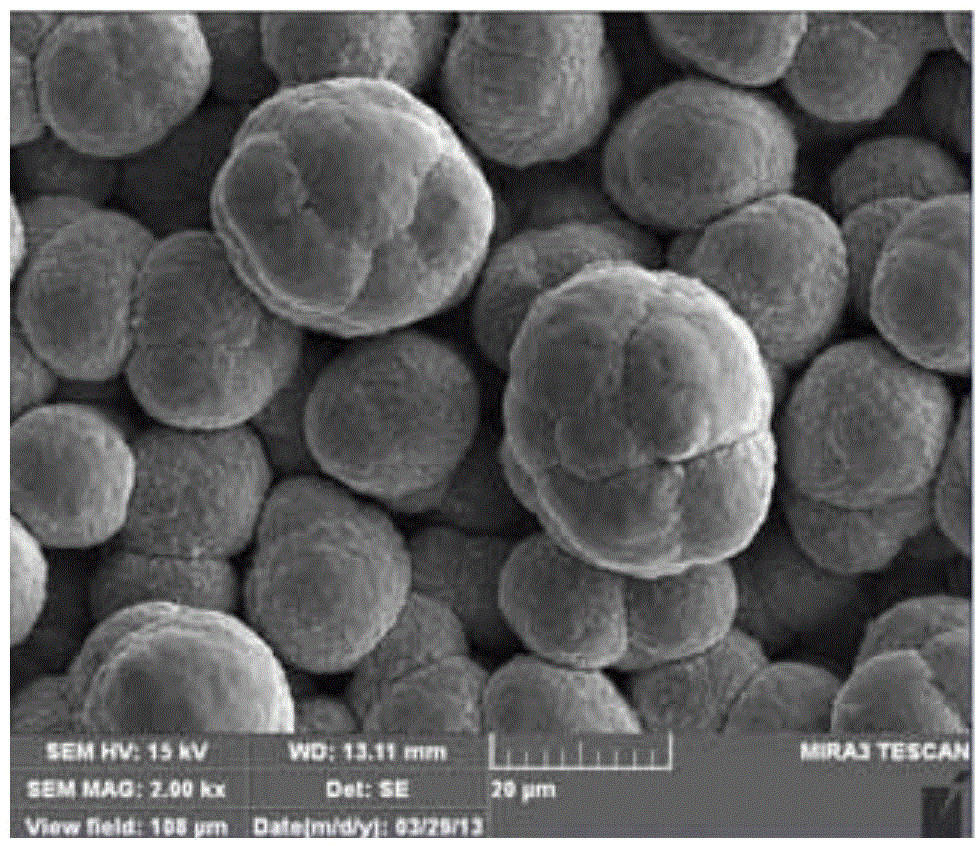
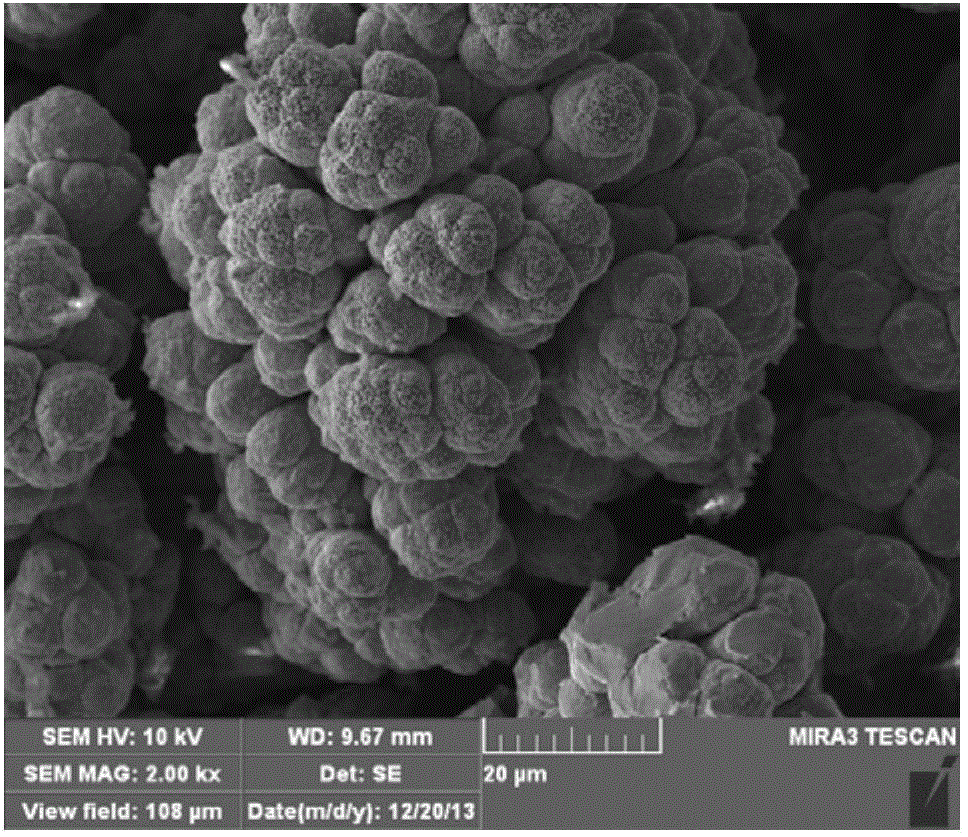
Preparation method for super-hydrophobic metal surface
A metal surface, super-hydrophobic technology, applied in the field of super-hydrophobic metal surface preparation, can solve the problems of single applicable substrate, poor surface binding force, complicated and time-consuming process, etc., to achieve enhanced film-base binding force, good binding, and controllable The effect of many conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037](1) Grind and polish the H62# brass plate with 800-mesh metallographic sandpaper to remove surface stains and defects, and then ultrasonically clean it with deionized water, acetone, 0.5mol / L dilute hydrochloric acid and deionized water for 5 minutes each.
[0038] (2) The pretreated brass plate is placed in a self-made electroplating tank (10cm×5cm×8cm) for constant current electroplating, the brass plate is used as the cathode plate, the red copper plate is used as the anode plate, and the plating solution is CuSO 4 ·5H 2 O and H 2 SO 4 The mixed solution, the concentration is: CuSO 4 ·5H 2 O125g / L, concentrated H 2 SO 4 55ml / L, current density 0.1A / cm 2 , the temperature is 26°C, the distance between the plates is 5cm, and the time is 20min.
[0039] (3) After the electroplating is completed, place the electroplated metal plate in deionized water and ultrasonically clean it for 5 minutes, and then place the plated area vertically along the wall surface under th...
Embodiment 2
[0042] (1) Grind and polish the H62# brass plate with 800-mesh metallographic sandpaper to remove surface stains and defects, and then ultrasonically clean it with deionized water, acetone, 1mol / L dilute hydrochloric acid and deionized water for 5 minutes each.
[0043] (2) Place the pretreated brass plate in a self-made electroplating tank (10cm×5cm×8cm) for pulse electroplating, the brass plate is used as the cathode plate, the red copper plate is used as the anode plate, and the plating solution is CuSO 4 ·5H 2 O180g / L, concentrated H 2 SO 4 50ml / L, the power supply is programmable DC power supply DP1116A (RIGOL), the loop current is controlled by electronic load IT8511 (ITECH), the current conversion cycle is 14s, and the current density in the first 8s of each cycle is 0.16A / cm 2 , the last 6s is 0.08A / cm 2 , the temperature is 25°C, the distance between the plates is 5cm, and the electroplating time is 20min.
[0044] (3) After the electroplating is completed, place ...
Embodiment 3
[0047] (1) The nickel plate was polished with 600-mesh metallographic sandpaper to remove surface stains and defects, and then ultrasonically cleaned with deionized water, ethanol, 1mol / L dilute hydrochloric acid and deionized water for 5 minutes each.
[0048] (2) Place the pretreated nickel plate in a self-made electroplating tank (10cm×5cm×8cm) pulse electroplating, the nickel plate is used as the cathode plate, the copper plate is used as the anode plate, and the plating solution is CuSO 4 ·5H 2 O160g / L, H 2 SO 4 60ml / L, the power supply is programmable DC power supply DP1116A (RIGOL), the loop current is controlled by electronic load IT8511 (ITECH), the current conversion cycle is 12s, and the current density in each cycle is 0.14A / cm for the first 7s 2 , the last 5s is 0.08A / cm 2 , the temperature is 28°C, the distance between the plates is 6cm, and the electroplating time is 18min.
[0049] (3) After the electroplating is completed, place the nickel plate in deioniz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com