A method for synthesizing carbon-coated lithium iron pyrophosphate by hydrothermal method
A carbon-coated technology for lithium ferrous pyrophosphate and lithium ferrous pyrophosphate, which is used in electrical components, electrochemical generators, battery electrodes, etc. It can achieve the effects of excellent electrochemical performance, fine powder and short synthesis period.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
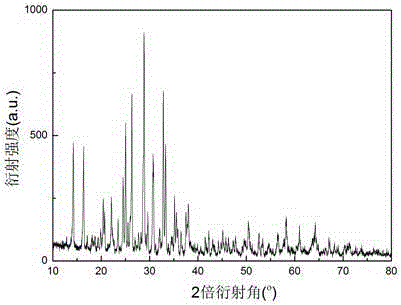
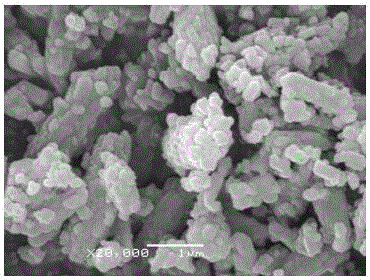
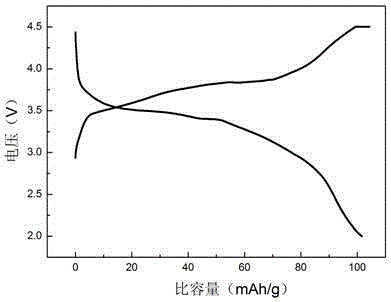
Image
Examples
Embodiment 1
[0035] This embodiment includes the following steps:
[0036] (1) This example is designed to generate 0.01mol of the target product Li 2 FeP 2 o 7 , prepare 100ml of the solution; weigh 0.01mol of citric acid, add it into a beaker with 40ml of deionized water, put the beaker into a 50°C constant temperature water bath, and stir magnetically; weigh 0.005mol of ferric sulfate (Fe 2 (SO 4 ) 3 ) and 0.04mol of lithium hydroxide, measure 0.02mol of orthophosphoric acid, add them into three small beakers with 20ml of deionized water respectively, stir until they are all dissolved, add the solutions containing iron source and lithium source in turn into the solution with the carbon source dissolved, then add ammonia water to adjust the pH of the mixed solution to 6, and then add the solution containing the phosphorus source to the mixed solution, then the iron ion concentration in the iron source is 0.1mol L -1 , magnetically stir the mixed solution in a water bath at 50°C for ...
Embodiment 2
[0043] This embodiment includes the following steps:
[0044] (1) This example is designed to generate 0.1mol of the target product Li 2 FeP 2 o 7 , prepare 1000ml of solution; weigh 0.1mol of ascorbic acid, add it into a beaker with 400ml of deionized water, put the beaker into a 60°C constant temperature water bath, and stir evenly with a mixer; weigh 0.1mol of ferric nitrate and 0.3mol of Lithium carbonate, measure 0.2mol of ammonium dihydrogen phosphate, add to three small beakers with 200ml of deionized water respectively, and stir until all of them are dissolved; Then add ammonia water to adjust the pH of the mixed solution to 5, then add the solution containing the phosphorus source to the mixed solution, then the iron ion concentration in the iron source is 0.1mol L -1 , magnetically stir the mixed solution in a water bath at 80°C for 1 h;
[0045] (2) Pour the mixed solution into an integrated temperature-controlled stirring reactor. The volume of the reactor is 1...
Embodiment 3
[0049] This embodiment includes the following steps:
[0050] (1) This example is designed to generate 0.05mol of the target product Li 2 FeP 2 o 7 , prepare solution 1000ml; Weigh 0.06mol polyethylene glycol, add in the beaker that has 550ml deionized water, put the beaker in 60 ℃ constant temperature water bath, stir evenly with agitator; Iron and 0.25mol of lithium acetate, measure 0.11mol of ammonium dihydrogen phosphate, respectively add to three small beakers with 150ml of deionized water, and stir until they are completely dissolved; the solutions containing iron source and lithium source are sequentially Add to the solution with carbon source dissolved, then add ammonia water to adjust the pH of the mixed solution to 4, then add the solution with phosphorus source to the mixed solution, then the iron ion concentration in the iron source is 0.05mol L -1 , magnetically stir the mixed solution in a water bath at 80°C for 2 hours;
[0051] (2) Pour the mixed solution i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com