Pharmaceutical composition and purpose of compound amino acid injection 18AA-V
A technology of 18AA-V and compound amino acids, which is applied in drug combination, drug delivery, medical preparations containing active ingredients, etc., and can solve problems such as unusable diseases, acid-base imbalance, and adverse problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0251] Embodiment 1, the preparation of compound amino acid injection (18AA-V), prescription:
[0252]
[0253] Preparation Process:
[0254] Weigh each raw material and auxiliary material according to the prescription; add 8000ml of water for injection into the dispensing tank, heat to boil, repeatedly vacuumize and replace with nitrogen to reduce the oxygen content in the dispensing tank, and then fill the tank with nitrogen during the whole process. Add raw and auxiliary materials sequentially between 96°C and 85°C: xylitol, antioxidant (sodium bisulfite), L-tyrosine, L-leucine, L-isoleucine, L-valine , L-methionine, stir until fully dissolved, and put L-phenylalanine, L-glutamic acid, and L-asparagine into the above-mentioned liquid mixing tank in sequence when the liquid temperature drops to 85°C°-65°C amino acid, L-lysine acetate, L-threonine, glycine, L-arginine, L-alanine, L-proline, L-serine, stir until completely dissolved, and wait until the solution temperatur...
Embodiment 2
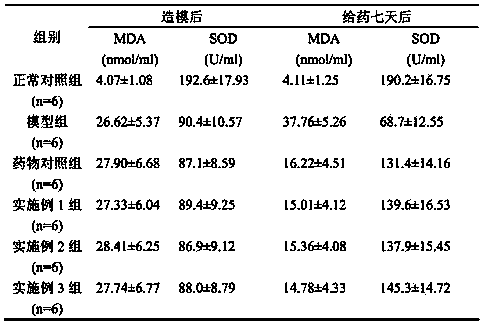
[0255] Refer to drug standard WS 1 -(X-324)-2003Z measures the content of each amino acid and xylitol in the pharmaceutical composition of the present invention that has been kept at room temperature for 3 months, and finds that they are all within the scope of 90-110% of the marked amount of the prescription; According to the determination, the sodium ion content in the solution is lower than 25mmol / L. In addition, the properties of the solution, insoluble particles, light transmittance, pH value, etc. are in line with the Chinese Pharmacopoeia 2010 version or the drug standard WS 1 - Regulations of (X-324)-2003Z. Embodiment 2, the preparation of compound amino acid injection (18AA-V), prescription:
[0256]
[0257]
[0258] Preparation Process:
[0259] Weigh each raw material and auxiliary material according to the prescription; add 7500ml of water for injection into the dispensing tank, heat and boil, and repeatedly adopt vacuum pumping and nitrogen filling repla...
Embodiment 3
[0261] Embodiment 3, the preparation of compound amino acid injection (18AA-V), prescription:
[0262]
[0263]
[0264] Preparation Process:
[0265] Weigh each raw material and auxiliary material according to the prescription; 1), add the prescribed amount of sorbitol into a batching bucket, add 1000ml of water for injection, stir to dissolve, add 0.3 g of activated carbon, stir, heat and boil for 20 minutes, and cool to 65-80°C , nitrogen flow for 20 minutes, stand-by; 2), add 7000ml of water for injection in the concentrated mixing tank, fill nitrogen while heating, add antioxidant (edetate calcium sodium, citric acid) when 95-90 ℃, stir Make it dissolve, then add L-tyrosine, L-leucine, L-isoleucine, L-valine, methionine stirring and dissolving of recipe quantity; 3), under nitrogen protection condition, Cool the solution obtained in step 2) to 65-75°C, add prescription quantities of L-phenylalanine, L-glutamic acid, L-aspartic acid, L-lysine acetate, L-threonine, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com