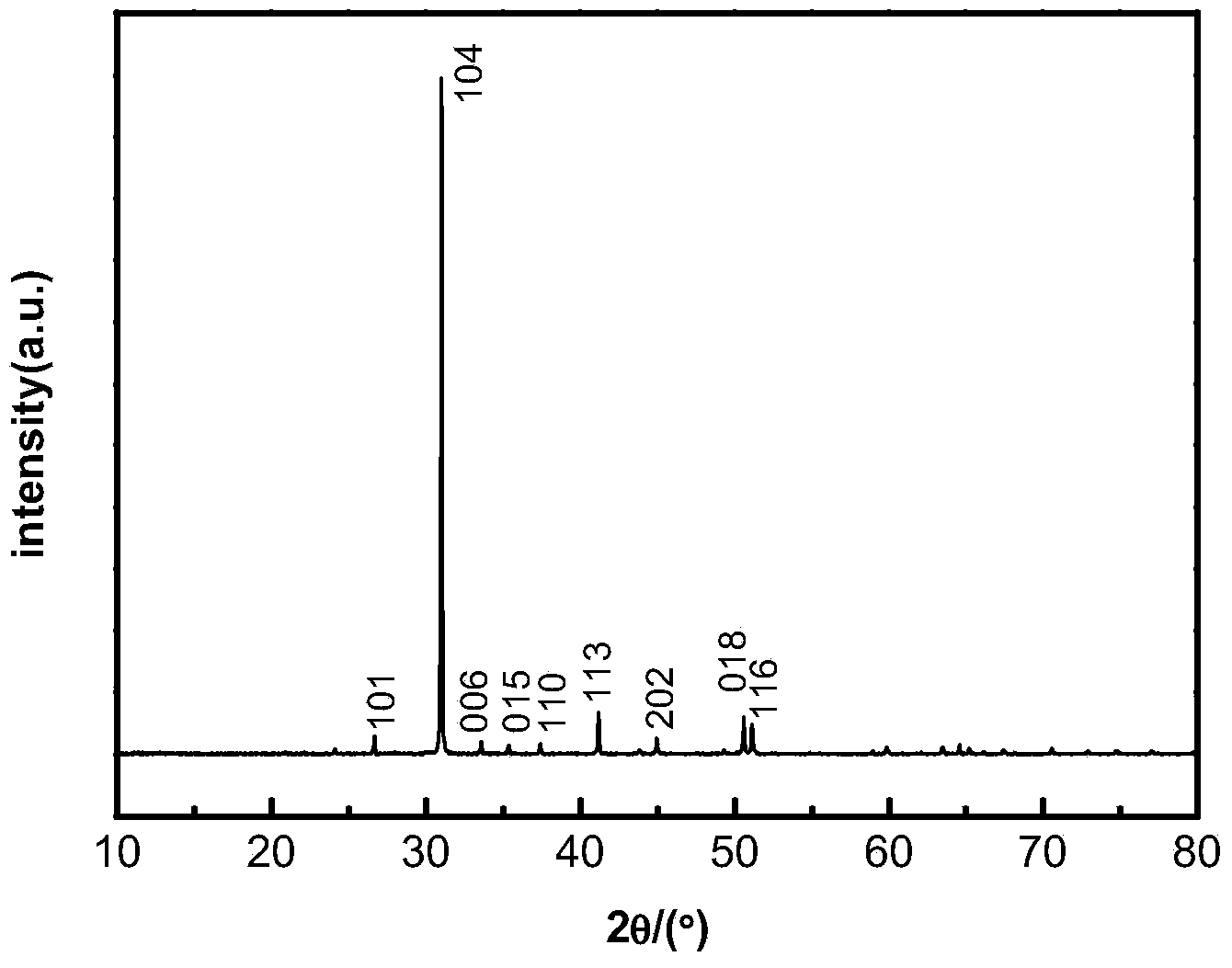
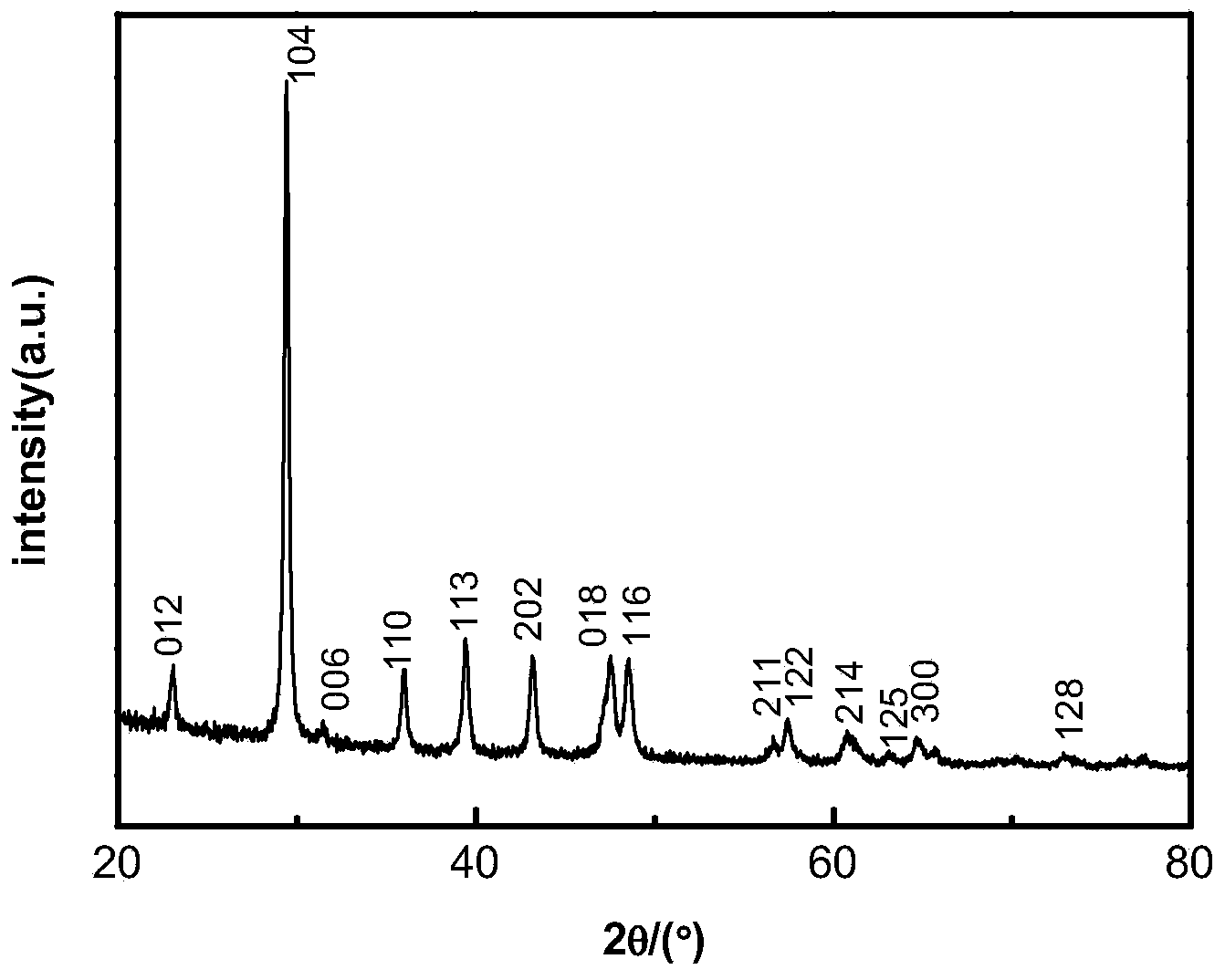
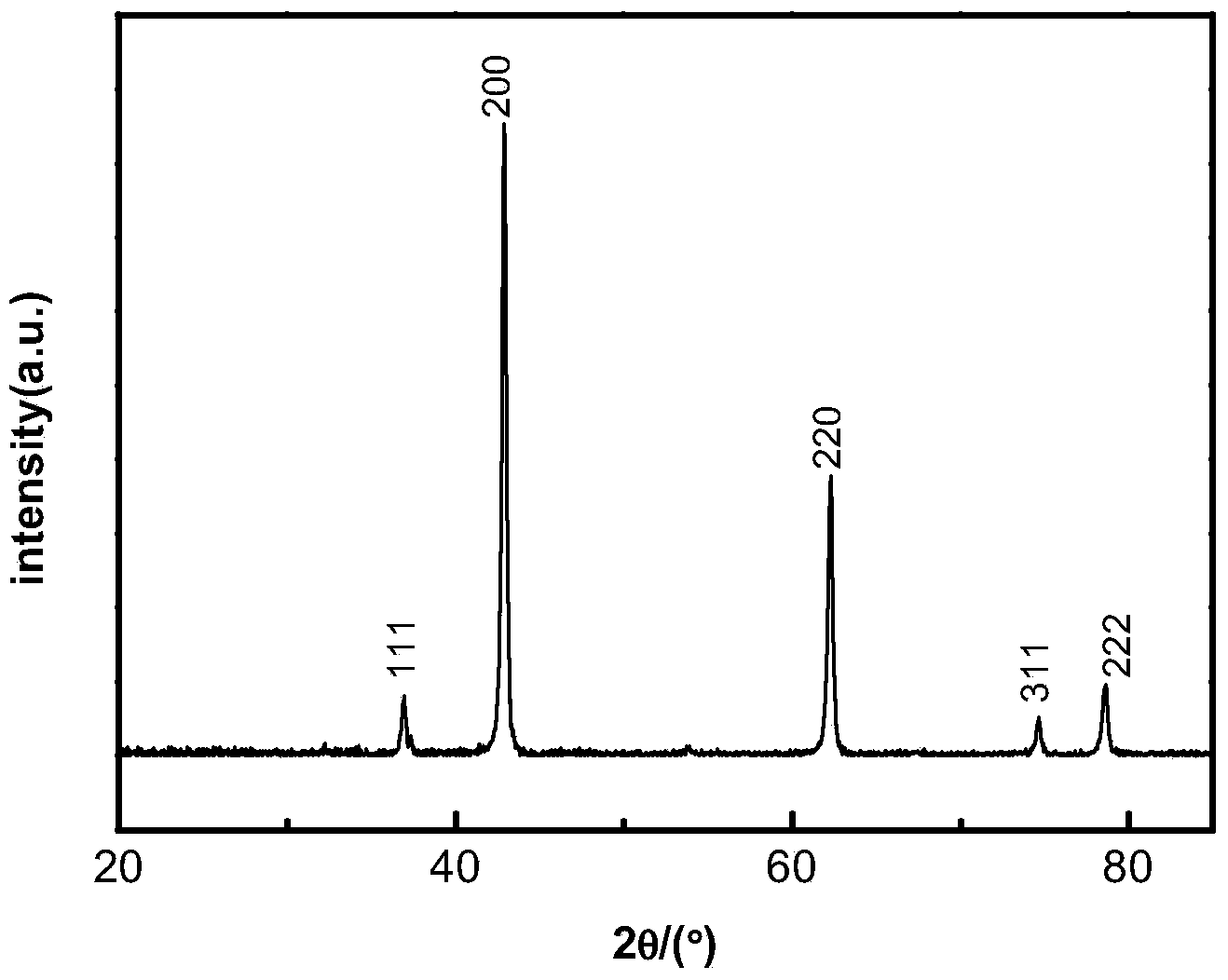
Method for preparing light calcium carbonate and magnesium oxide from dolomite
A light calcium carbonate, dolomite technology, applied in the directions of magnesium oxide, calcium carbonate/strontium/barium, etc., can solve the problems of reducing the operability of workers, reducing the added value of products, reducing factory income, etc., and achieving good industrial application prospects , easy to control, avoid the effect of secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The method for preparing light calcium carbonate and magnesium oxide by dolomite in the present embodiment is to operate as follows:
[0054] a. Crushing the dolomite raw ore to a particle size of 0.5-5mm, placing it in a muffle furnace and calcining at 950°C for 1.5h to obtain dolomite lime powder, which is sealed and stored;
[0055] b. Place the dolomite lime powder obtained in step a into water at 80°C for digestion, the liquid-solid ratio is 20mL:1g, stir and react at a constant temperature for 30min, and the rotating speed is 400r / min, cool after the reaction, and age for 24h to obtain the digestate;
[0056] c. Add sodium gluconate solution to the digestive juice obtained in step b, the molar ratio of sodium gluconate to calcium in the digestive juice is 1:1, stir and react at a constant temperature at 25°C for 0.7 hours, and the speed is 400r / min; the reaction is over Afterwards, soluble calcium ion solution and filter cake are obtained by filtration and separat...
Embodiment 2
[0064] The method for preparing light calcium carbonate and magnesium oxide by dolomite in the present embodiment is to operate as follows:
[0065] a. Crushing the dolomite raw ore to a particle size of 0.5-5mm, placing it in a muffle furnace and calcining at 950°C for 1.5h to obtain dolomite lime powder, which is sealed and stored;
[0066] b. Place the dolomite lime powder obtained in step a in water at 85°C for digestion, the liquid-solid ratio is 20mL:1g, stir and react at a constant temperature for 40min, and the rotating speed is 400r / min, cool after the reaction, and age for 24h to obtain the digestive juice;
[0067] c. Add sucrose solution to the digestive solution obtained in step b. The molar ratio of sucrose to calcium in the digestive solution is 1.5:1. Stir and react at a constant temperature of 30°C for 0.8 hours at a speed of 500r / min; Soluble calcium ion solution and filter cake are obtained by filtering and separating;
[0068] d. Stir and heat the soluble ...
Embodiment 3
[0074] The method for preparing light calcium carbonate and magnesium oxide by dolomite in the present embodiment is to operate as follows:
[0075] a. Crushing the dolomite raw ore to a particle size of 0.5-5mm, placing it in a muffle furnace and calcining at 950°C for 1.5h to obtain dolomite lime powder, which is sealed and stored;
[0076] b. Place the dolomite lime powder obtained in step a in water at 90°C for digestion, the liquid-solid ratio is 20mL:1g, stir and react at a constant temperature for 60min, and the rotating speed is 400r / min, cool after the reaction, and age for 24h to obtain the digestive juice;
[0077] c. Add the sodium citrate solution to the digestive solution obtained in step b, the molar ratio of sodium citrate to calcium in the digestive solution is 4:1, stir and react at a constant temperature of 35°C for 1 hour, and the rotation speed is 300r / min; the reaction ends Afterwards, soluble calcium ion solution and filter cake are obtained by filtratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com