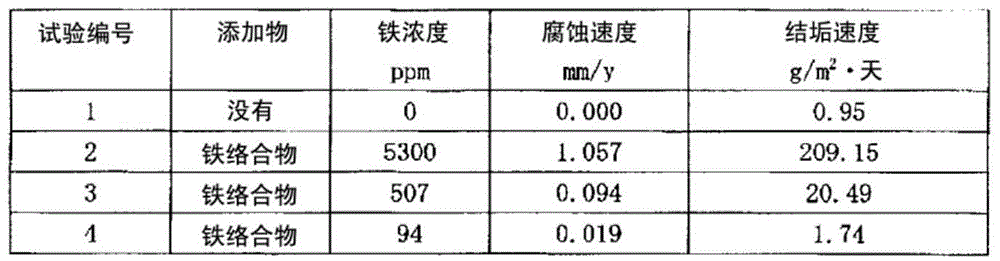
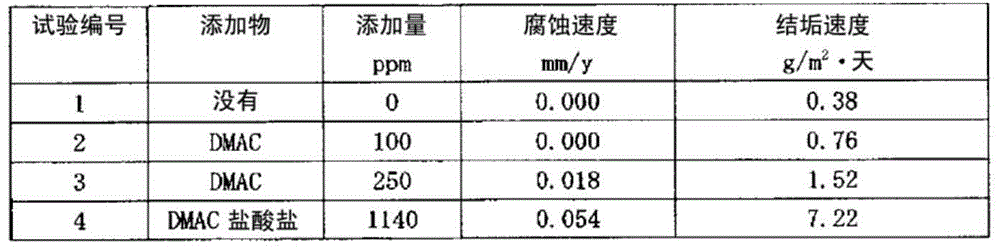
Production method for 1-chloro-3,3,3-trifluoropropene
A technology of trifluoropropene and its manufacturing method, which is applied in the direction of organic chemical methods, chemical instruments and methods, preparation of halogenated hydrocarbons, etc., can solve problems such as difficulty in obtaining raw materials and difficult to become quantitative reactions, and achieve the effect of inhibiting corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0088] Weigh 300 g of activated alumina (NKH3-24 manufactured by Sumitomo Chemical: particle size 2 to 4 mm, specific surface area 340 m 2 / g), wash with water to remove the powder attached to the surface. 115 g of hydrogen fluoride (hydrofluoric anhydride) was dissolved in 1035 g of water to prepare a 10% hydrogen fluoride aqueous solution. The prepared 10% hydrogen fluoride aqueous solution was slowly poured into the cleaned activated alumina, stirred and allowed to stand for 3 hours, washed with water, filtered, and then dried in an electric furnace at 200°C for 2 hours. 150 cc of dry activated alumina was put into a stainless steel reaction tube with an inner diameter of 1 inch and a length of 30 cm, and the electric furnace was heated to 200°C while flowing nitrogen gas, and hydrogen fluoride treatment was performed while hydrogen fluoride and nitrogen gas were introduced together. The flow rates of nitrogen and hydrogen fluoride were adjusted in such a way that the temp...
Embodiment 1
[0090] Manufacture of 1,1,1,3,3-pentachloropropane
[0091] Into a 1000 ml glass autoclave equipped with a stirrer, 3.2 mol of carbon tetrachloride, 0.8 mol of chloroform, 0.06 mol (1.25 mol% relative to carbon tetrachloride) of N,N-dimethylacetamide (DMAC) were charged. , 0.02 mol (relative to carbon tetrachloride is 0.625 mol%) iron powder, after replacing the air in the reactor with nitrogen, seal it, and heat to 140° C. while stirring at 250 rpm for 30 minutes. The pressure at this time was 0.25 MPaG (gauge pressure, hereinafter the same in this specification). After 30 minutes, vinyl chloride was pressurized to approximately 0.33 MPaG, and 2 moles of vinyl chloride were added so as to maintain the pressure as the reaction progressed. The reaction time was 160 minutes.
[0092] After the reaction was terminated, the reactor was left to cool, and the contents were taken out, and the metal salts were removed, and analyzed by gas chromatography.
[0093] The yield of tar...
Embodiment 2
[0095] Manufacture of 1,1,1,3,3-pentachloropropane
[0096] Reaction, recovery, and analysis were performed in the same manner as in Example 1 except that 0.06 mol of hexamethylphosphoric triamide was used instead of DMAC. The time required for the reaction was 180 minutes. As a result, the yield of target 1,1,1,3,3-pentachloropropane was 54.4 mol % with respect to the charged carbon tetrachloride. In addition, the remaining carbon tetrachloride was 1.22 mol (38.1% with respect to the charged amount). On the other hand, the vinyl chloride reaction rate and the 1,1,1,3,3-pentachloropropane selectivity at this time were 96.8% and 93.1%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com