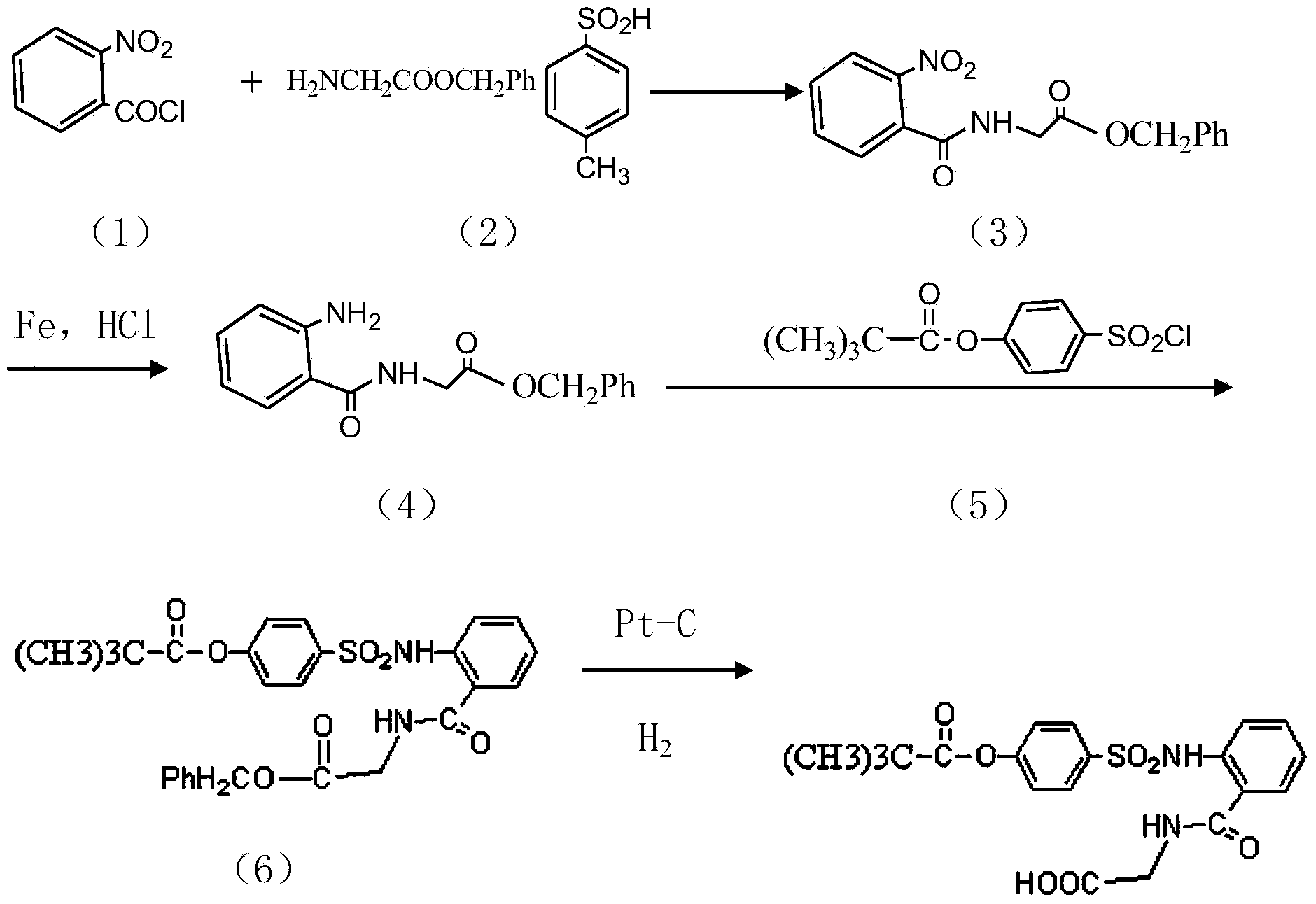
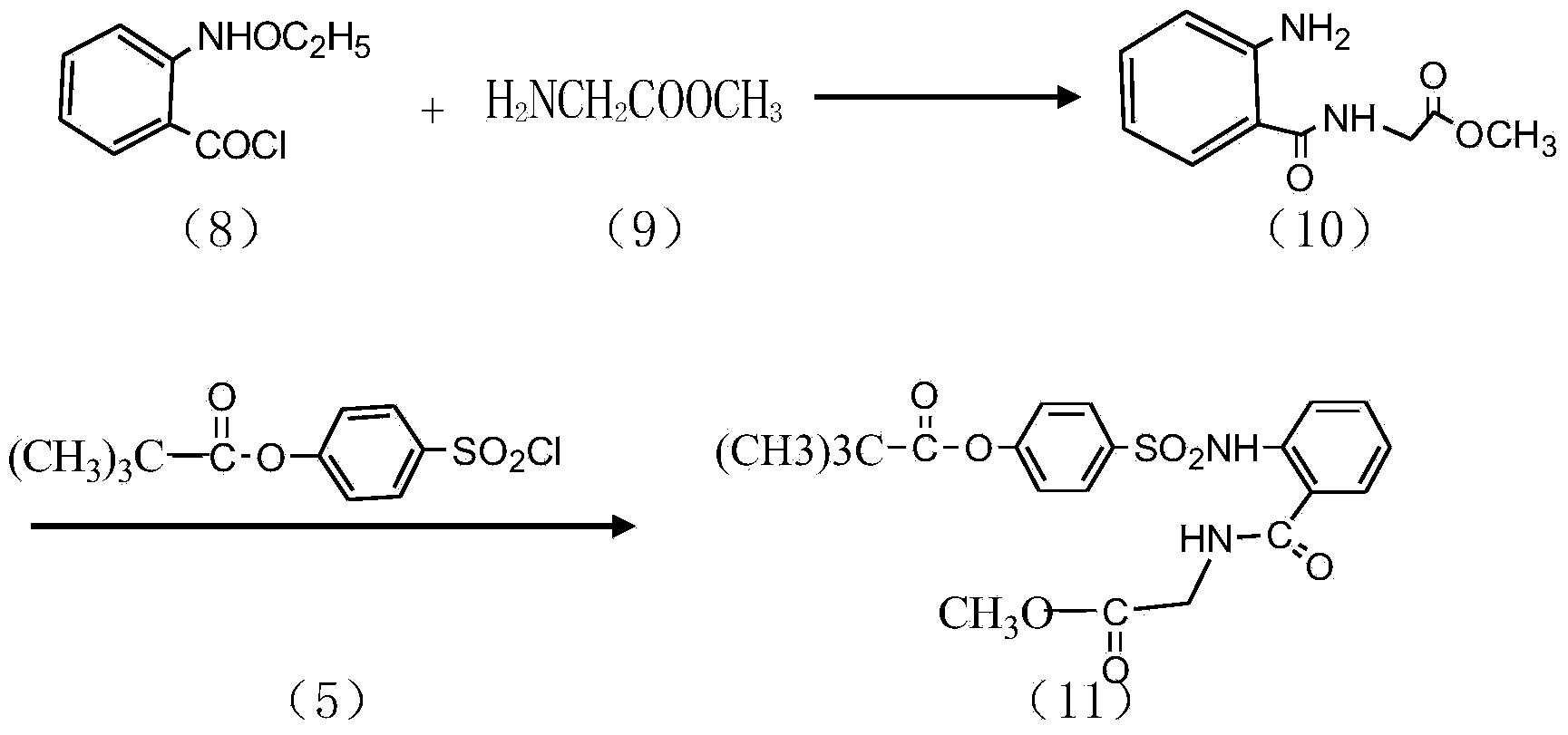
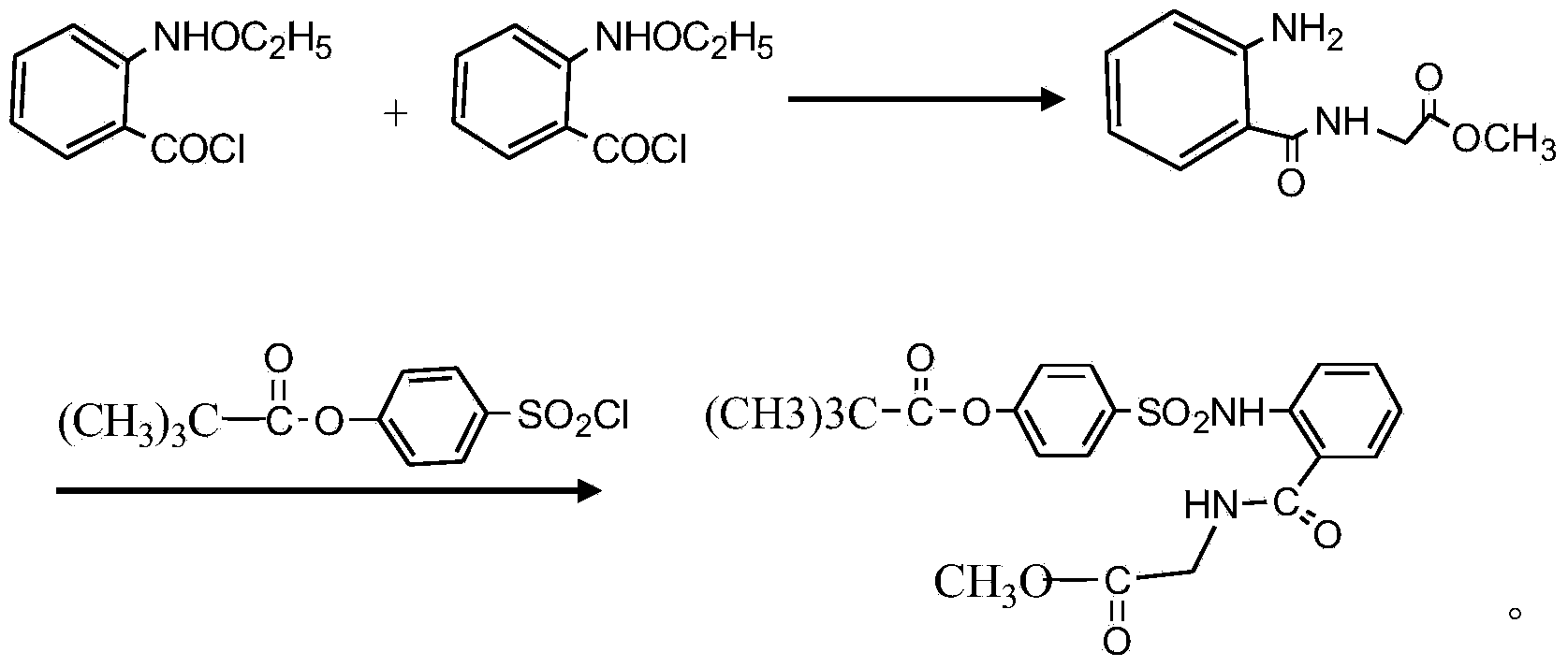
Preparation method of sivelestat sodium hydrate intermediate
A technology of severestat sodium and intermediates, applied in the preparation of sulfonic acid amides, organic chemistry and other directions, can solve the problems of low industrial production safety factor, low iron powder reduction yield, easy poisoning of palladium carbon, etc. Synthetic process route, simple solvent recycling, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A kind of preparation method of sivelestat sodium intermediate that the embodiment of the present invention 1 proposes comprises the following steps:
[0022] Take 1 mol of acid chloride (acyl chloride is obtained by reacting with thionyl chloride after acylation with amino benzoic acid as raw material), 500 ml of non-polar organic solvent, 1.0-3.0 mol of alkali acid-binding agent, glycine methyl ester hydrochloride Put 1.0-2.0mol and appropriate amount of amide catalyst into the reaction container in turn, and react at 25-30°C for 5-7h; pour the reaction product into 300ml of hydrochloric acid with a mass fraction of 18-20wt%, and stir at 20-30°C React for 1.8-2.5h; After the reaction is finished, let stand to separate the organic layer, the water layer is N-benzyloxycarbonylmethyl-2-aminobenzamide glycine solution, and adjust the pH value of the water layer with dilute alkali until PH=7. After stirring for a period of time, re-measure the pH value to remain unchanged,...
Embodiment 2
[0025] A kind of preparation method of sivelestat sodium intermediate that the embodiment of the present invention 2 proposes, comprises the steps:
[0026] S1. Put 50 grams of acid chloride, 500 ml of dichloromethane, 65 g of triethylamine, 35 g of glycine methyl ester hydrochloride, and an appropriate amount of amide catalyst into the reaction container in turn, and react at 28 ° C for 6 hours; after the reaction, pour the reaction product into In 300ml of hydrochloric acid with a mass fraction of 20wt%, stir and react at 25°C for 2h; let stand, separate the organic layer, the water layer is N-benzyloxycarbonylmethyl-2-aminobenzamide glycine solution, and the water layer is washed with dilute Adjust the pH value with alkali until PH = 7. After stirring for a period of time, re-measure the pH value to remain unchanged, filter out the light yellow solid, recrystallize with absolute ethanol after drying, and dry to obtain a off-white solid as N-benzyloxycarbonylmethyl Base-2-am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com