Method for producing 16-dehydropregnenolone acetate
A technology of dienool ketone acetate and production method, applied in the directions of steroids, organic chemistry, etc., can solve the problems such as unrecoverable catalyst, complicated production process, increased cost, etc., so as to improve the utilization rate of raw materials and the yield of products. , the effect of reducing equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
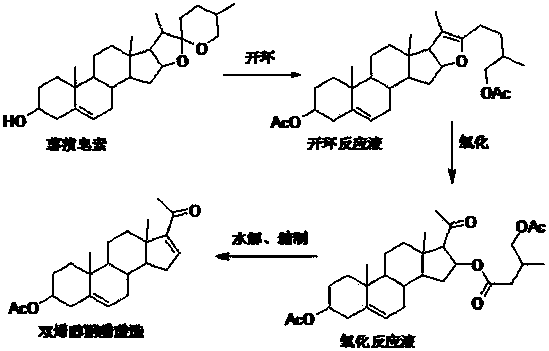
[0030] Take 4g of diosgenin and place it in a 100ml three-necked reaction flask, add 30ml of acetic anhydride and 18ml of glacial acetic acid, heat and stir on the electric heating mantle to dissolve, continue heating and reflux for 2h, stop heating, cool to room temperature, and obtain 52.4g of ring-opening reaction solution.
[0031] Put 52.4g of the ring-opening reaction solution into the bubbler, place the bubbler in an ice-water bath at about 10°C, and then pass in ozone, and continue the reaction for 1 hour. TLC will track the reaction until it is completely oxidized, and stop the introduction of ozone. The ozone is adsorbed by activated carbon, and 48.3 g of the oxidation reaction solution is taken out.
[0032] Add 20ml of water to 48.3g of the oxidation reaction solution, heat to 95°C and stir under reflux for 1.5h for hydrolysis, then distill the hydrolyzed solution to recover glacial acetic acid, then add 100ml of cyclohexane and petroleum ether to the still liquid (...
Embodiment 2
[0034] Take 35g of diosgenin and place it in a 1000ml three-necked reaction bottle, add 400ml of acetic anhydride and 80ml of glacial acetic acid, heat and stir on the electric heating mantle to dissolve, continue to heat and reflux for 1.5h, stop heating, cool to room temperature, and obtain 443.6g of ring-opening reaction solution .
[0035] Pass 443.6g of the ring-opening reaction liquid into the bubbler, place the bubbler in an ice-water bath at about -5°C, and then pass through ozone, and continue the reaction for 2.5 hours. TLC will track the reaction until it is completely oxidized, and then stop the introduction of ozone , excess ozone was adsorbed with active carbon, and 385.7 g of the oxidation reaction solution was taken out.
[0036] Add 200ml of water to 385.7g of the oxidation reaction solution, heat to 95°C and stir to reflux for 2 hours for hydrolysis, then distill the hydrolyzed solution to recover glacial acetic acid, then add 1000ml of cyclohexane to the sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com