A kind of ether-based thiourethane collector and its preparation and application method
A technology of thiourethane and collector, which is applied in the direction of solid separation and flotation, can solve the problem of no ether-based thiourethane, and achieve the effects of reduced dosage, effective separation, and strong collection capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The synthesis of embodiment 1 ether group thiourethane collector MCTC-1
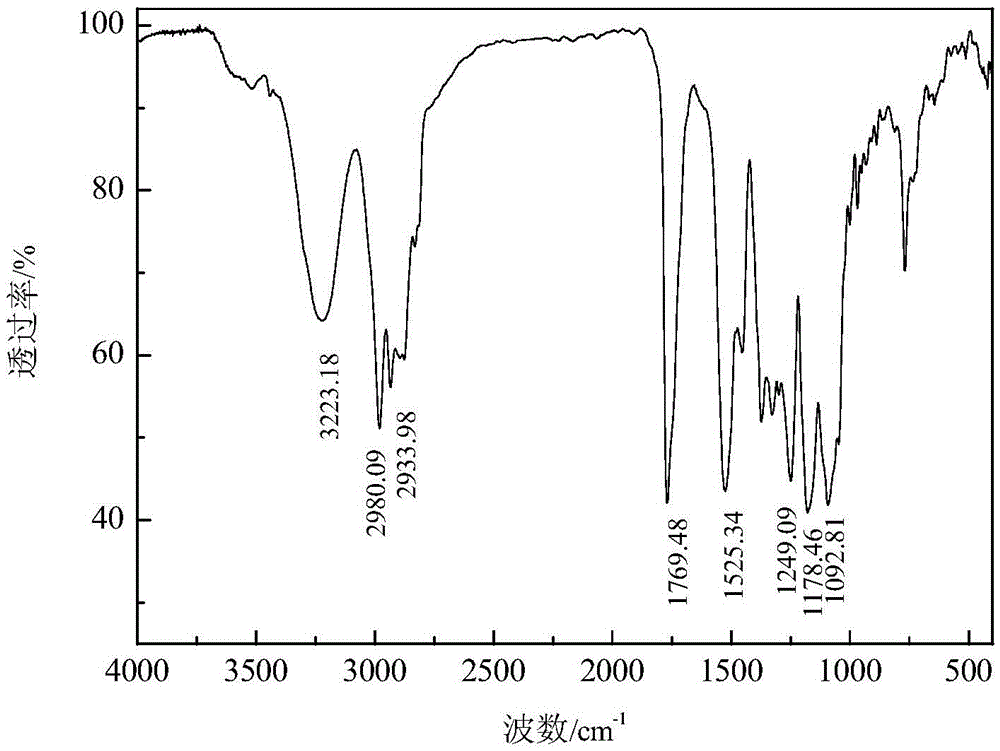
[0057] Add 65.6 parts of N-ethoxycarbonyl isothiocyanate intermediate to 54.1 parts of propylene glycol methyl ether, and stir at 60°C for 3 hours to obtain O-methoxyisopropyl-N-ethoxycarbonyl thiocarbamate and The product of propylene glycol methyl ether (code name: MCTC-1) has a product yield based on isothiocyanate of 85.34%, the content of ether-based thiourethane is 77.08%, and the content of propylene glycol methyl ether is 20.49%. After the product was separated and purified by silica gel column chromatography, O-methoxyisopropyl-N-ethoxycarbonylthiocarbamate was characterized. The pure product Mr: 221.27, and the MS detected by mass spectrometry: 221.8 [M+H] + , 243.9[M+Na] + . Infrared spectrum analysis and nuclear magnetic resonance analysis spectra are shown in figure 1 and Figure 11 , see Table 1 and Table 2 for the data.
Embodiment 2
[0058] The synthesis of embodiment 2 ether group thiourethane collector MCTC-2
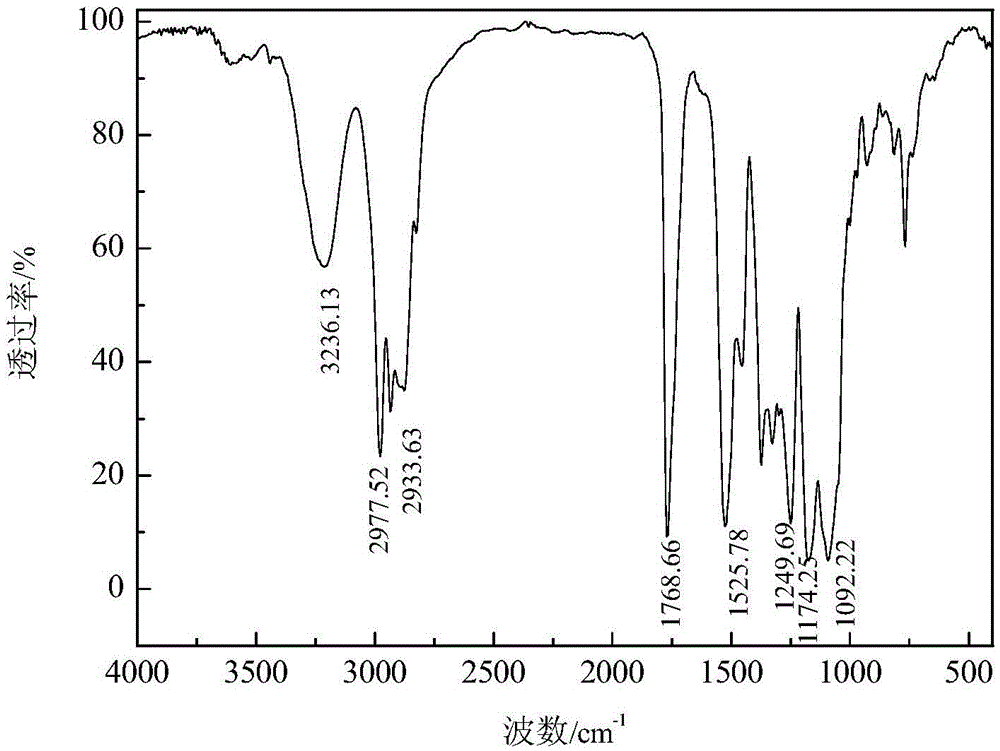
[0059] Add 65.6 parts of N-ethoxycarbonyl isothiocyanate intermediate to 88.9 parts of dipropylene glycol methyl ether, and stir at 70°C for 3 hours to obtain ) product of isopropyl-N-ethoxycarbonyl thiocarbamate and dipropylene glycol methyl ether (code name: MCTC-2), the product yield based on isothiocyanate is 80.06%, and the content of ether group thiocarbamate is 73.00% %, the dipropylene glycol methyl ether content is 24.14%. After the product was separated and purified by silica gel column chromatography, O-(2-methoxy-1-methylethoxy) isopropyl-N-ethoxycarbonylthiocarbamate was characterized. The pure product Mr: 279.35, after Mass Spectrometry MS: 279.7[M+H] + 、301.9[M+Na] + . Infrared spectrum analysis and nuclear magnetic resonance analysis spectra are shown in figure 2 and Figure 12 , see Table 1 and Table 2 for the data.
Embodiment 3
[0060] The synthesis of embodiment 3 ether group thiourethane collector MCTC-3
[0061] Add 65.6 parts of N-ethoxycarbonyl isothiocyanate intermediate to 123.8 parts of tripropylene glycol methyl ether, and stir at 60°C for 5 hours to obtain O-[2-(2-methoxy-1-methyl Ethoxy)-1-(methylethoxy)]isopropyl-N-ethoxycarbonylthiocarbamate and tripropylene glycol methyl ether (code: MCTC-3), isothiocyanate-based products The yield is 81.93%, the content of O-[2-(2-methoxy-1-methylethoxy)-1-(methylethoxy)]isopropyl-N-ethoxycarbonylthiocarbamate It is 71.77%, and the content of tripropylene glycol methyl ether is 26.33%. After the product was separated and purified by silica gel column chromatography, O-[2-(2-methoxy-1-methylethoxy)-1-(methylethoxy)]isopropyl-N-ethoxy Characterized by carbonyl thiourethane, the pure product Mr: 337, detected by mass spectrometry MS: 337.8 [M+H] + 、360[M+Na] + . Infrared spectrum analysis and nuclear magnetic resonance analysis spectra are shown in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com