Bisphenol A type poly sulfuric acid (ammonia) ester compound and synthetic method thereof
A technology of ester compounds and synthesis methods, applied in the field of polysulfate (ammonia) ester compounds, new type bisphenol A type polysulfate (ammonia) ester compounds and their synthesis, can solve difficult and no substantive basic reactions Break through and restrict the development and application of polyester materials, and achieve the effects of low environmental pollution, excellent mechanical properties, and good alkali resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
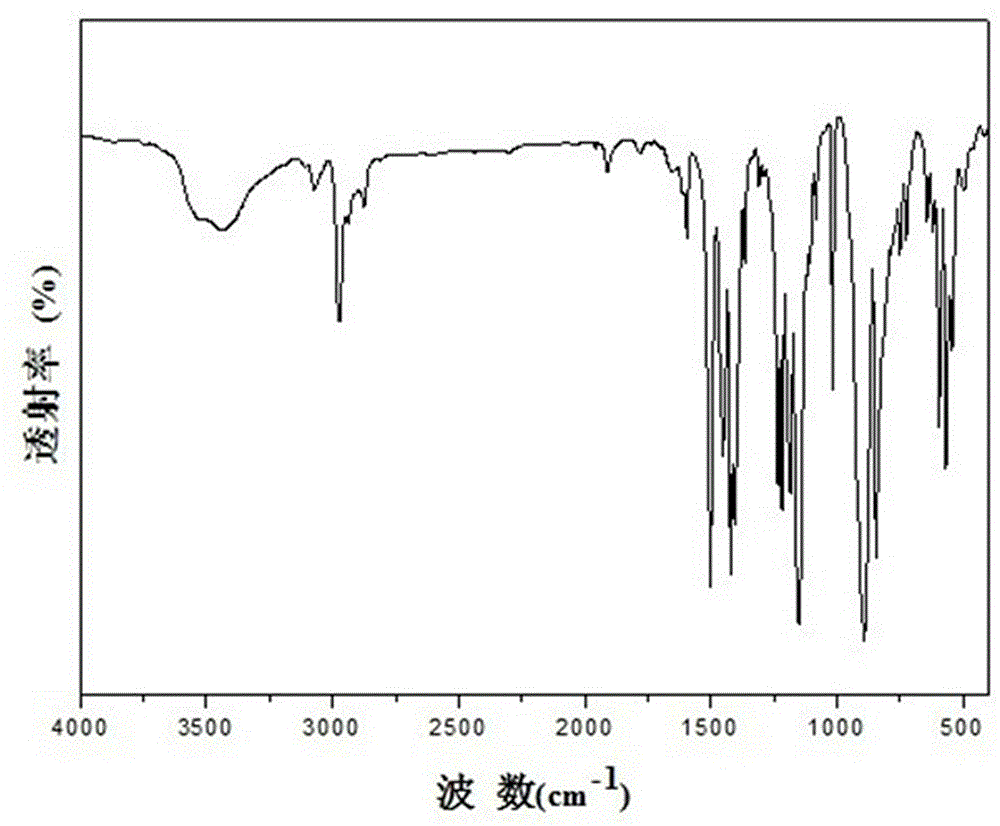
Image
Examples
Embodiment 1
[0043] Example 1、 Bisphenol A type polysulfate -1 with a degree of polymerization of 10~50 ( PSE-1 )Synthesis
[0044] (1) Synthesis of bisphenol A dioxysulfonyl fluoride: Dissolve 22.8 kg (100 mol) of bisphenol A (scientific name: 2,2-bis(4-hydroxyphenyl)propane) in 5 L of dichloromethane or chloroform Add 10.1 kg (100 mol) of triethylamine, and then carefully and continuously introduce sulfonyl fluoride gas. React at 25°C for 12 hours. After the reaction is complete, simply concentrate and filter to obtain 2,2-bis(4-oxysulfonyl fluorophenyl) propane as a white solid product of dioxysulfonyl fluoride (English) The full name is 4,4'-(propane-2,2-diyl)bis(4,1-phenylene) disulfofluoridate]) 38.5 kg, yield: 98%.
[0045] NMR analysis data of the synthesized product: 1 H NMR (300 MHz, CDCl 3 ): δ 7.24-7.32(m, 8 H), 1.70 (s, 6 H); 13 C NMR (75 MHz, CDCl 3 ): 150.4, 148.2, 128.7, 120.6, 42.9, 30.7; 19 F NMR (282 MHz, CDCl 3 ): δ +37.47;
[0046] High resolution mass spectrometry da...
Embodiment 2
[0058] Example 2 、 Bisphenol A polysulfate -1 with a degree of polymerization of 10~50 ( PSE-1 ) Solvent-free synthesis
[0059] (1) Synthesis of bisphenol A dioxysulfonyl fluoride: the same as in Example 1.
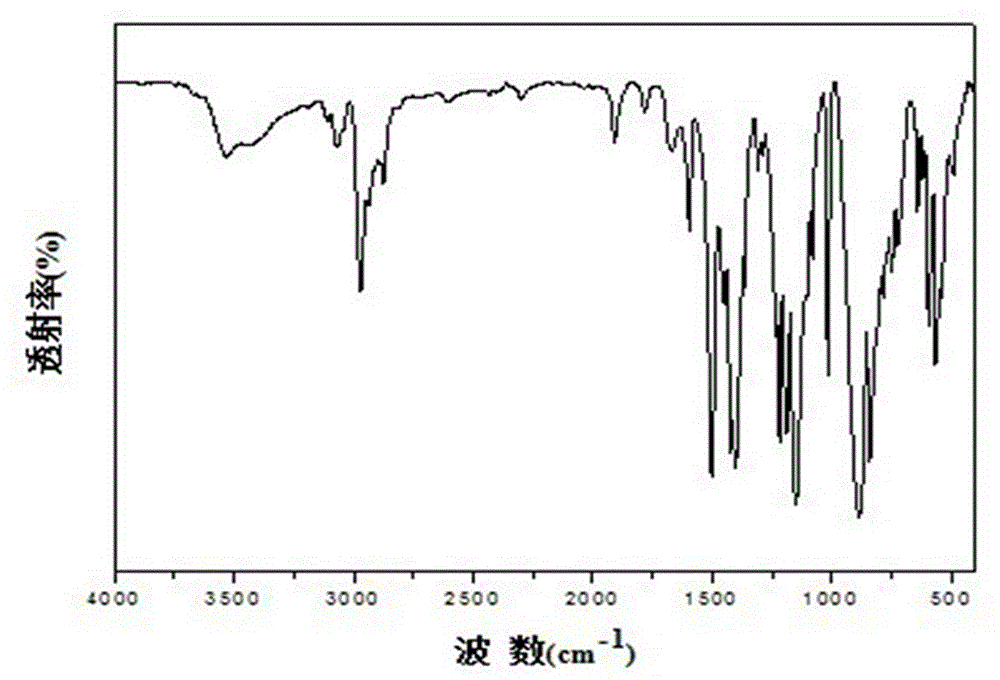
[0060] (2) Bisphenol A polysulfate -1 ( PSE-1 ) Synthesis: Under the protection of nitrogen, dissolve 3.92 kg (10 mol) of the synthesized bisphenol A dioxysulfonyl fluoride in an equal molar amount of trimethylsilyl protected bisphenol 3.73 kg (10 mol) liquid , Add 10% molar amount (1.0 mol) of the catalyst 1,8-diazabicyclo(5.4.0)undec-7-ene ( DBU ), react for 6 hours under mechanical stirring at 50°C, add water to the mixture to terminate the reaction, separate the precipitated polymer product, extract with ethanol and dry to obtain 5.31 g of bisphenol A polysulfate as a white solid, with a yield of 69 %. The infrared spectrum of the polymer is shown in Figure 1. The performance indicators of the polymerization product are as follows:
[0061] Weight average molecular we...
Embodiment 3
[0067] Example 3、 Bisphenol A type polysulfate-2 with a degree of polymerization of 200~300 ( PSE-2 )Synthesis
[0068] (1) Synthesis of bisphenol A dioxysulfonyl fluoride: the same as in Example 1.
[0069] (2) The degree of polymerization is 200~300 bisphenol A type polysulfate-1 ( PSE-2 )Synthesis
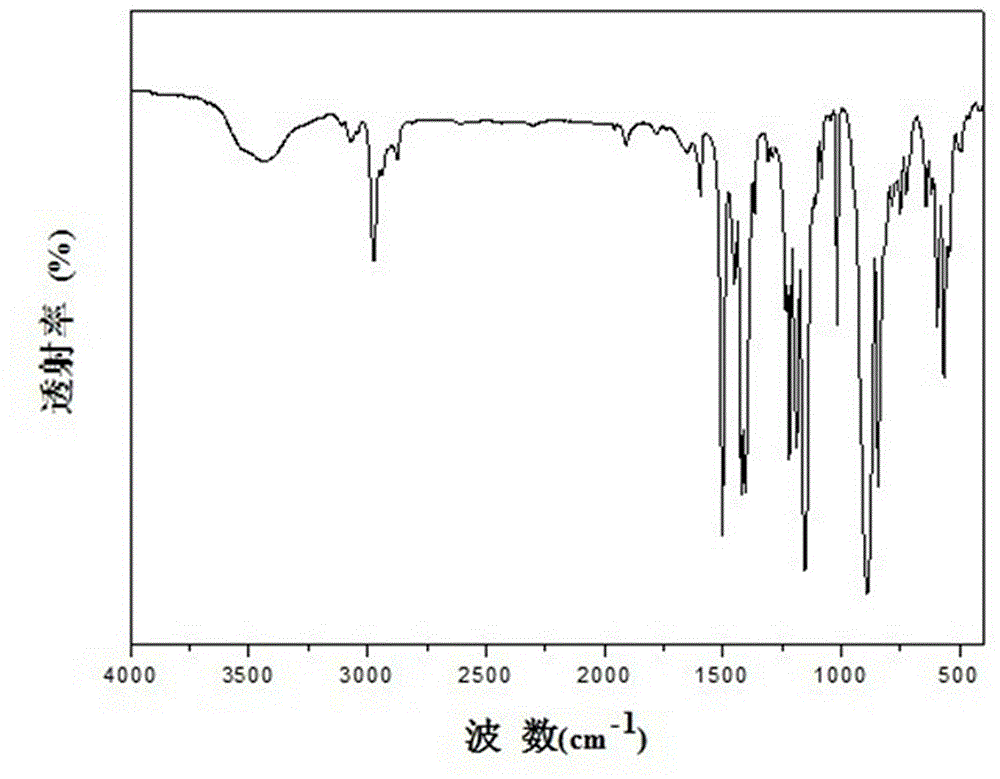
[0070] Under the protection of nitrogen, dissolve 3.92 kg (10 mol) of bisphenol A dioxysulfonyl fluoride prepared in step (1) and 3.73 kg (10 mol) of trimethylsilyl-protected bisphenol in 500 mL of N- Methylpyrrolidone ( NMP ), add 10% molar (1.0 mol) of catalyst DBU (1,8-diazabicyclo(5.4.0)undec-7-ene), react for 12 hours under mechanical stirring at 25℃, then pour the mixture into water to terminate the reaction, separate the precipitated polymer product, and extract it with ethanol After extraction and drying, 6.38 g of white solid bisphenol A polysulfate was obtained with a yield of 83%. Figure 2 shows the infrared spectrum of the polymer. The performance indicators of the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com