Method for detecting drug and enantiomer impurities of drug
A technology for enantiomers and impurities, which is applied in the field of simultaneous detection of drugs and their enantiomer impurities, can solve problems such as no reports of high-performance liquid chromatography analysis methods, and achieves short running time, loose requirements, and detection. Finished low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (a) Pre-column derivation:
[0030] Dissolve 10mg of S-Xemilofiban hydrochloride in 8ml of dichloromethane, react with 0.5ml of triethylamine and 15mg of p-nitrobenzoyl chloride at room temperature for 60min, add 0.2ml of diethylamine, place it for 20min, and place it in a water bath at 60°C The solvent was evaporated to dryness, and the residue was dissolved in absolute ethanol and adjusted to 20ml (concentration of about 0.5mg / ml) as the raw material for the test solution. Take an appropriate amount of R-Xemilofiban hydrochloride reference substance according to the law to prepare a solution with a concentration of about 10 μg / ml as the enantiomer positioning solution. In addition, S-Xemilofiban hydrochloride reference substance and R-Xemilofiban hydrochloride reference substance were prepared according to the law to prepare solutions with concentrations of 0.5mg / ml and 2.5μg / ml respectively as system suitability solutions.
[0031] b) HPLC analysis and UV detection:...
Embodiment 2
[0036] Embodiment 1 methodological verification result
[0037] 1. Limit of detection and limit of quantitation
[0038] A signal-to-noise ratio of 3:1 was used as the detection limit, and a signal-to-noise ratio of 10:1 was used as the lowest quantification limit.
[0039] Component
The lowest concentration limit of quantification (μg / ml)
Minimum detection limit concentration (μg / ml)
S-Xemilofiban derivatives
0.0503
0.0201
R-Xemilofiban derivatives
0.0513
0.0205
[0040] 2. Linearity and range
[0041] According to embodiment 1 chromatographic conditions, inject liquid chromatograph respectively, record chromatogram, carry out linear regression to peak area (Y) by solution concentration (X), get regression equation
[0042]
[0043]
[0044] 3. Accuracy
[0045] Weigh about 10.0 mg of 9 parts of S-Xemilofiban, prepare the test solution according to the derivatization method, and add 0.25%, 0.5%, and 0.75% of the ...
Embodiment 3
[0061] Isolation of S-Xemilofiban hydrochloride composition
[0062] (a) Pre-column derivation:
[0063] Dissolve 10 mg of S-Xemilofiban hydrochloride for injection (containing excipients such as mannitol) (calculated as S-Xemilofiban hydrochloride) in 10 ml of absolute ethanol, centrifuge, and evaporate the supernatant to dryness. Dissolve the residue in 8ml of dichloromethane, add 0.5ml of triethylamine and 15mg of p-nitrobenzoyl chloride, react at room temperature for 60min, add 0.2ml of diethylamine, let stand for 20min, evaporate the solvent to dryness in a water bath at 60°C, and use Dissolve in absolute ethanol and set the volume to 20ml preparation need testing solution. Another prescription amount of blank excipients was prepared according to law as a blank excipient solution.
[0064] b) HPLC analysis and UV detection:
[0065] With embodiment 1.
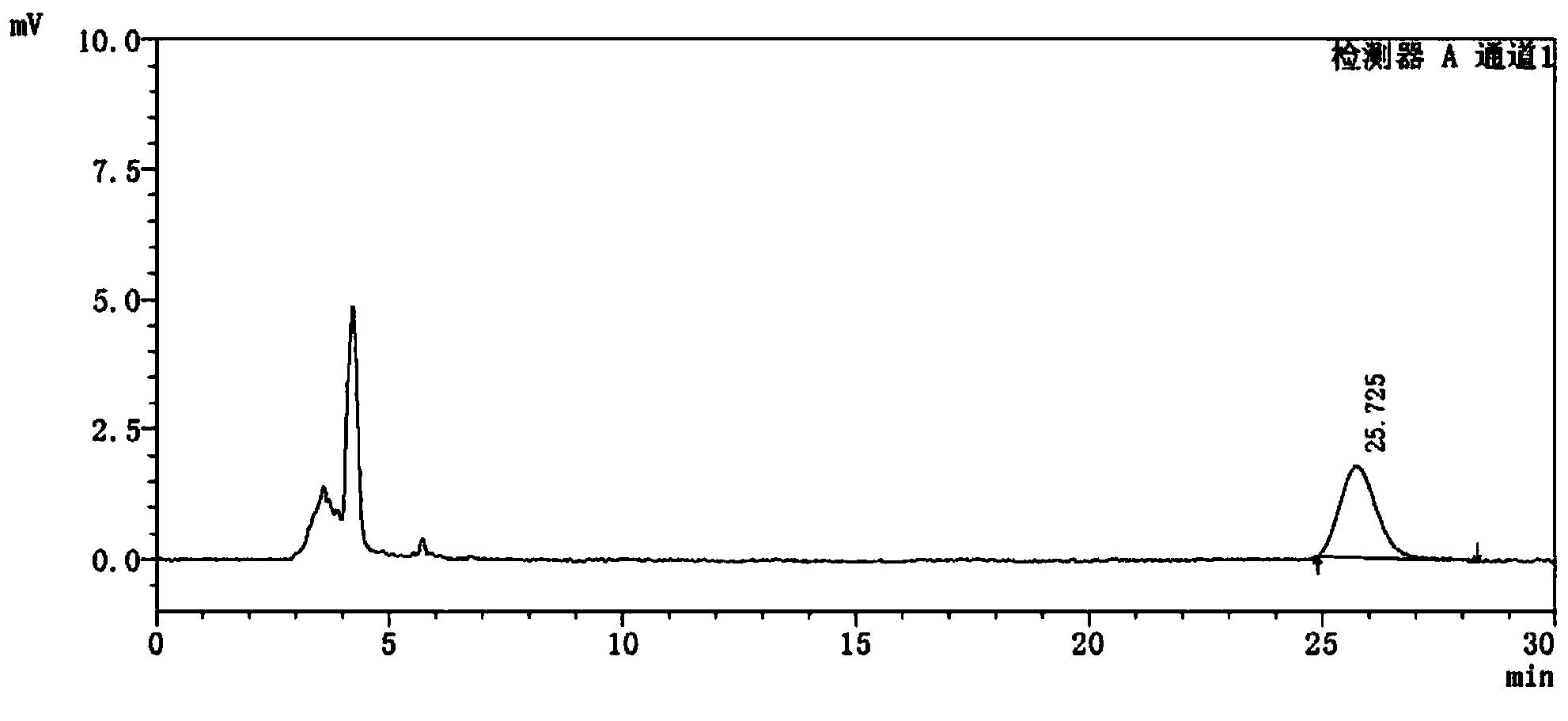
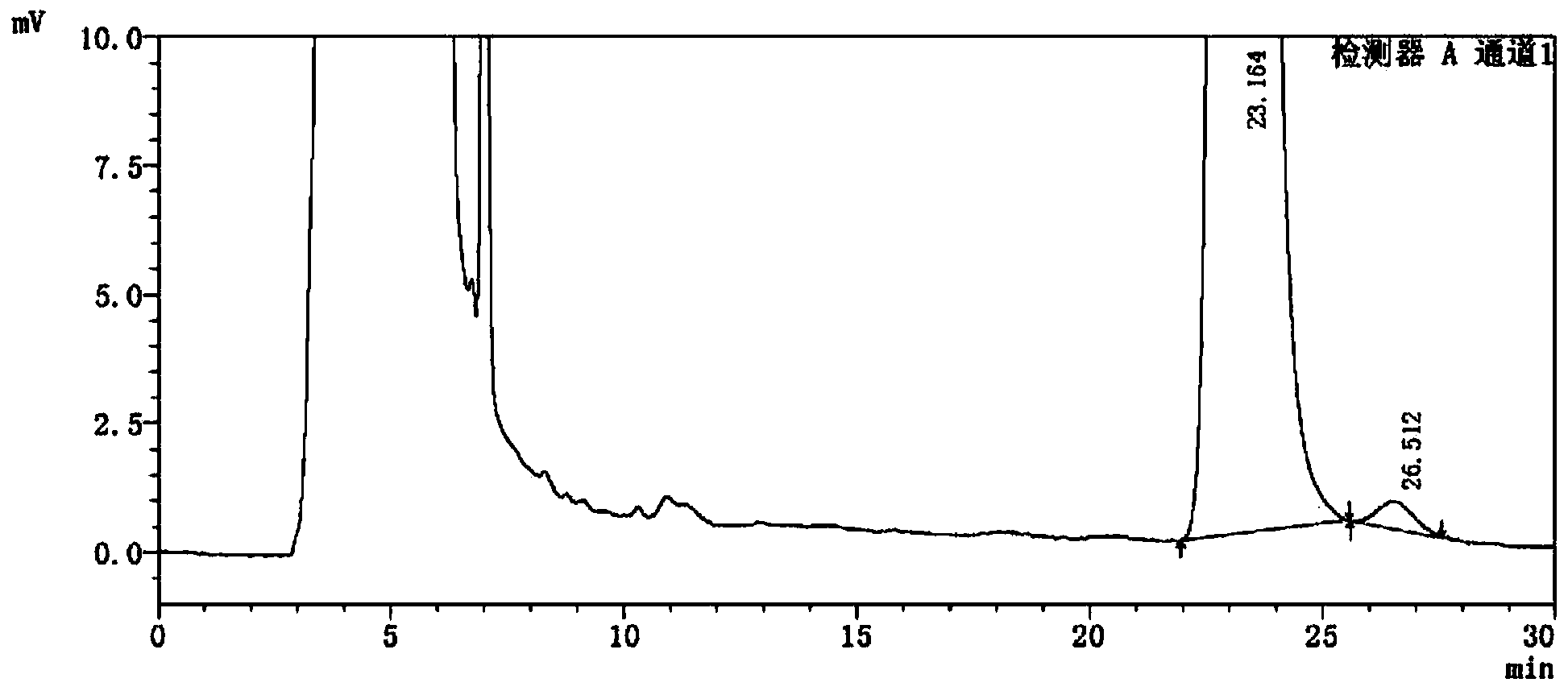
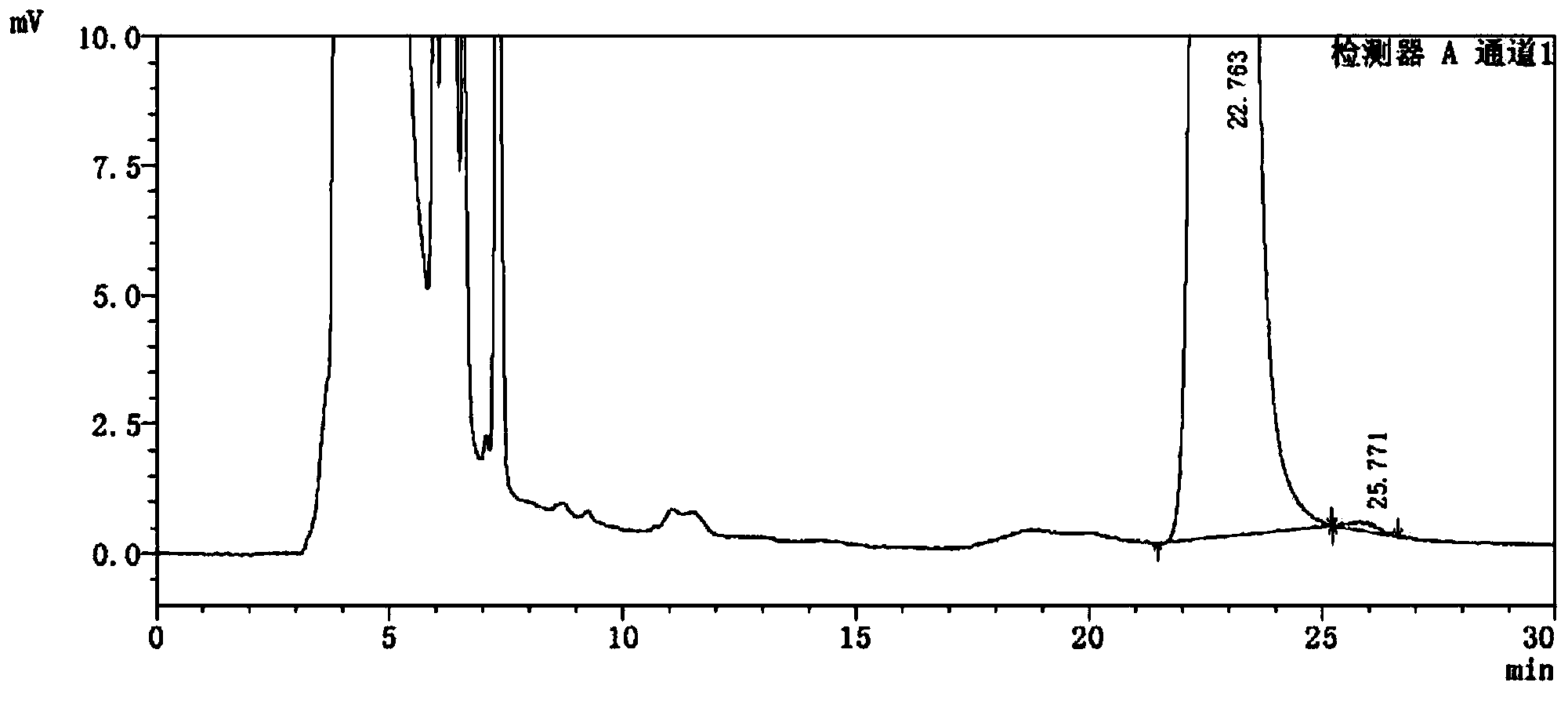
[0066] see attached results Figure 4 with 5 .
[0067] Figure 4 with Figure 5 Display: the blank excipient s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com