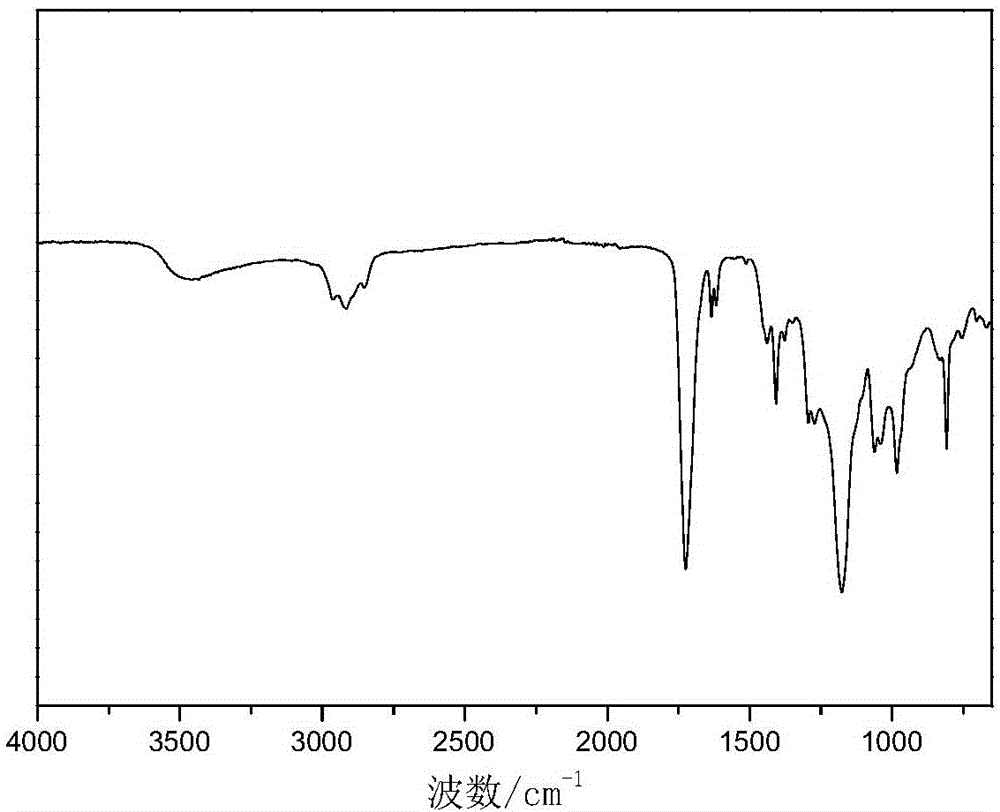
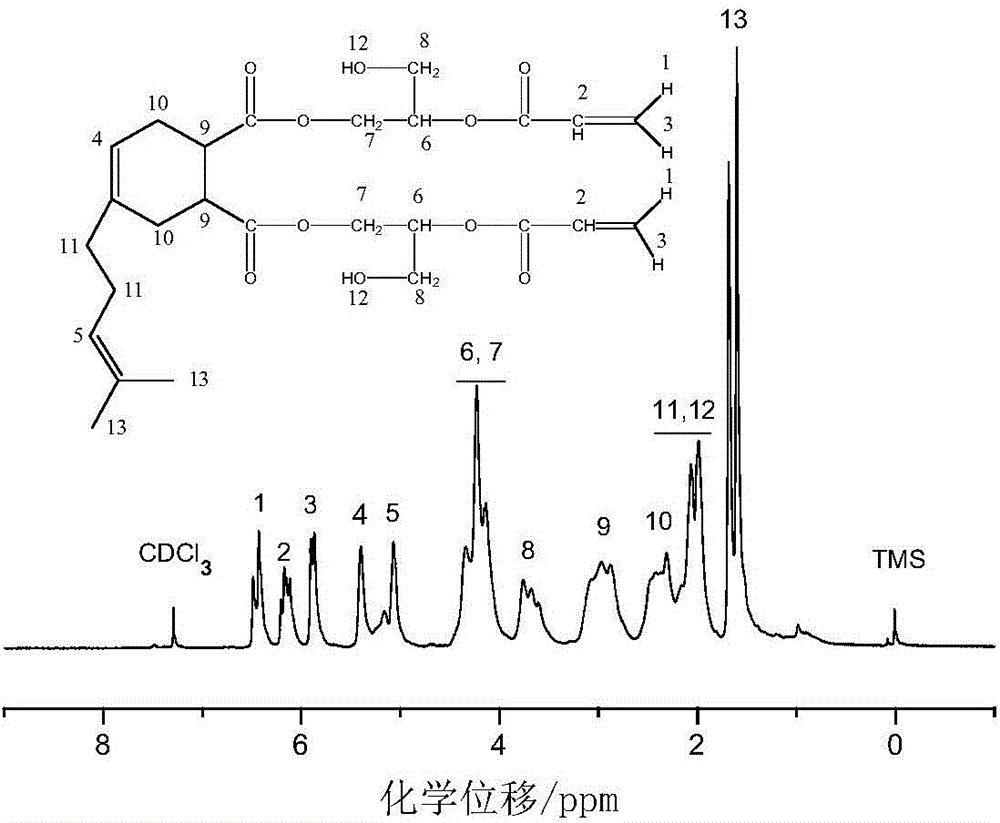
Method for preparing myrcenyl vinyl ester resin monomer
A technology of alkenyl vinyl ester and resin monomer, which is applied in the field of preparation of unsaturated resin monomer, can solve the problems of high content of toxic components, poor weather resistance, etc., and achieves good product stability, strong corrosion resistance, and synthetic process Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Add maleic anhydride to a four-neck bottle, raise the temperature to 50°C to dissolve it completely, add dropwise myrcene with a molar ratio of 1.1:1 to maleic anhydride, and keep the temperature at 50~60°C during the dropping process ℃. After the dropwise addition, react at 70° C. for 5 h to obtain myrcene maleic anhydride.
[0031] (2) Put equimolar water and acid anhydride into the reactor, and add epichlorohydrin whose mole number is 10 times the number of acid anhydride moles, and stir at 100°C for two hours to fully hydrolyze the acid anhydride group. Then add benzyltriethylammonium chloride accounting for 1 wt% of the total mass of the reactants, and react at 100°C for 2h.
[0032] (3) Cool down to 60°C, add sodium hydroxide and calcium oxide with an equal molar number of carboxyl groups, and stir at 60°C for 3 hours. Spread filter paper and diatomaceous earth, and filter under reduced pressure at a vacuum degree of 0.1Mpa to obtain a filtrate. Rotary evap...
Embodiment 2
[0036] (1) Add acrylic acid to a four-necked bottle, raise the temperature to 50°C, add myrcene with a molar ratio of 1:1 to acrylic acid dropwise, and keep the temperature at 50-60°C during the dropwise addition. After the dropwise addition, react at 60° C. for 5 h to obtain myrcenyl acrylic acid.
[0037] (2) Add epichlorohydrin whose molar number is 10 times that of carboxylic acid, tetramethylammonium chloride accounting for 1 wt% of the total mass of reactants, and react at 100°C for 2 hours.
[0038] (3) Cool down to 60°C, add sodium hydroxide and calcium oxide with an equal molar number of carboxyl groups, and stir at 60°C for 3 hours. Spread filter paper and diatomaceous earth, and filter under reduced pressure at a vacuum degree of 0.1Mpa to obtain a filtrate. Rotary evaporation removes excess epichlorohydrin to obtain a myrcene-based epoxy resin intermediate.
[0039] (4) Add the myrcene-based epoxy resin intermediate and N,N-dimethylbenzylamine and 0.1wt% of the p...
Embodiment 3
[0042] (1) Add methacrylic acid to a four-neck flask, raise the temperature to 50°C, add myrcene with a molar ratio of 1.2:1 to methacrylic acid dropwise, and maintain the temperature at 50-60°C during the dropwise addition. After the dropwise addition, react at 70° C. for 5 h to obtain myrcenyl methacrylic acid.
[0043] (2) Add epichlorohydrin whose mole number is 10 times that of carboxylic acid, tetramethylammonium bromide accounting for 2.0 wt% of the total mass of reactants, and react at 100°C for 2 hours.
[0044] (3) Cool down to 60°C, add sodium hydroxide ion and calcium oxide having an equal molar number to the carboxyl group, and stir at 60°C for 3 hours. Spread filter paper and diatomaceous earth, and filter under reduced pressure at a vacuum degree of 0.1Mpa to obtain a filtrate. Rotary evaporation removes excess epichlorohydrin to obtain a myrcene-based epoxy resin intermediate.
[0045](4) Add the myrcene-based epoxy resin intermediate and N,N-dimethylbenzylam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com