Mn<4+>-doped red light-emitting material and preparation method thereof as well as novel lighting source
A technology of red light-emitting and light-emitting materials, applied in the fields of light-emitting materials, chemical instruments and methods, electrical components, etc., can solve the problems that the emission wavelength cannot meet the requirements and the cost of raw materials is high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The present invention also provides a kind of Mn 4+ A method for preparing a doped red luminescent material, comprising the steps of:
[0031] Step 1: according to chemical formula A 14-y B 6 C 10-x o 35 :xMn 4+ ,yM 3+ Stoichiometric ratio, weigh the compound containing A, the compound containing B, the compound containing C, the compound containing Mn and the compound containing M, grind and mix uniformly to obtain the mixture;
[0032] Step 2: roasting the mixture obtained in Step 1 to obtain phosphor;
[0033] Step 3: Grinding and dispersing the phosphor powder obtained in Step 2 to obtain Mn 4+ Doped red luminescent material.
[0034] The A-containing compound described in Step 1 of the present invention is preferably an A-containing oxide, nitrate, hydroxide, halide or carbonate; the A-containing oxide is more preferably CaO, SrO or BaO; The nitrate containing A is more preferably Ca(NO 3 ) 2 , Sr(NO 3 ) 2 or Ba(NO 3 ) 2 ; The hydroxide containing A ...
Embodiment 1
[0049] Choose calcium carbonate, aluminum oxide, zinc oxide, and manganese carbonate as starting materials, according to the molar ratio of each element Ca:Zn:Al:Mn=14:6:9.7:0.3, corresponding to x=0.3, y=0, weighed respectively Get four kinds of raw materials, control raw material mixture gross weight to be 20 grams. Carry out thorough grinding and mixing, put it into an alumina crucible, put it into a high-temperature furnace, and bake it in the air at 1200 ° C for 3 hours, take it out when it is cooled to room temperature, and obtain a kind of Mn after grinding and dispersing. 4+ Doped red light material, its composition is Ca 14 Zn 6 al 9.7 mn 0.3 o 35 .
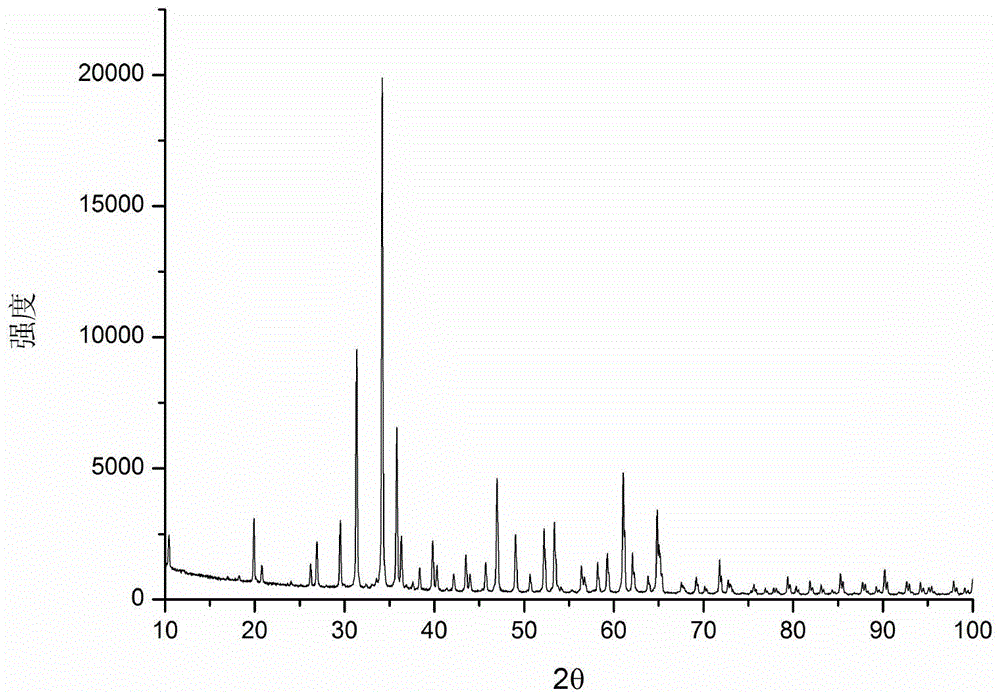
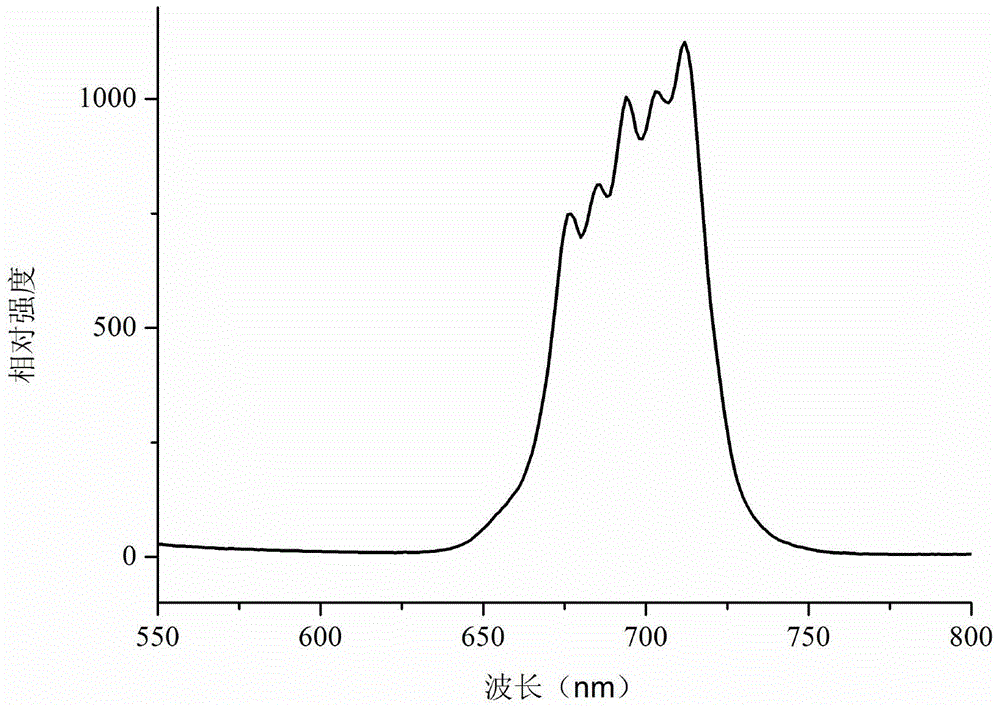
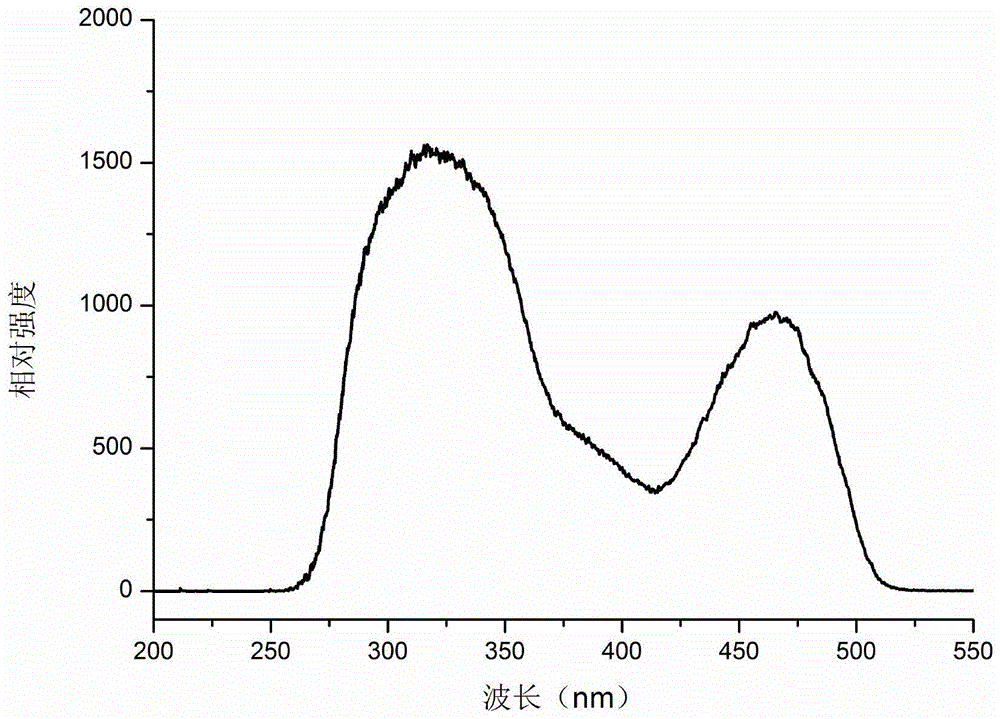
[0050] figure 1 For the Mn obtained by Example 1 of the present invention 4+ The XRD pattern of the doped red light material, as can be seen from the figure, the spectrum is consistent with Ca 14 Zn 6 al 10 o 35 Consistent, proving that the Ca 14 Zn 6 al 9.7 mn 0.3 o 35 . figure 2 For the Mn obtained by...
Embodiment 2
[0052] Select calcium carbonate, aluminum oxide, zinc oxide, and manganese carbonate as starting materials, according to the molar ratio of each element Ca:Zn:Al:Mn=14:6:9:1, corresponding to x=1, y=0, respectively weighed Get four kinds of raw materials, control raw material mixture gross weight to be 20 grams. Carry out thorough grinding and mixing, put it into an alumina crucible, put it into a high-temperature furnace, and bake it in the air at 1150°C for 10 hours, take it out when it is cooled to room temperature, and obtain a kind of Mn after grinding and dispersing. 4+ Doped red light material, its composition is Ca 14 Zn 6 al 9 MnO 35 . The fluorescent spectral properties of the red luminescent material are similar to those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| color rendering index | aaaaa | aaaaa |
| color rendering index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com