Method for fast screening mammal cell strain having high exogenous protein expression level
A mammalian and cell technology, applied in the direction of cells modified by introducing foreign genetic material, introducing foreign genetic material using a vector, recombinant DNA technology, etc., can solve the problems of restricting large-scale production of recombinant protein drugs, time-consuming and cost, etc. Achieve the effect of saving development cost and time, reducing the probability of false positive clones, and improving screening efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
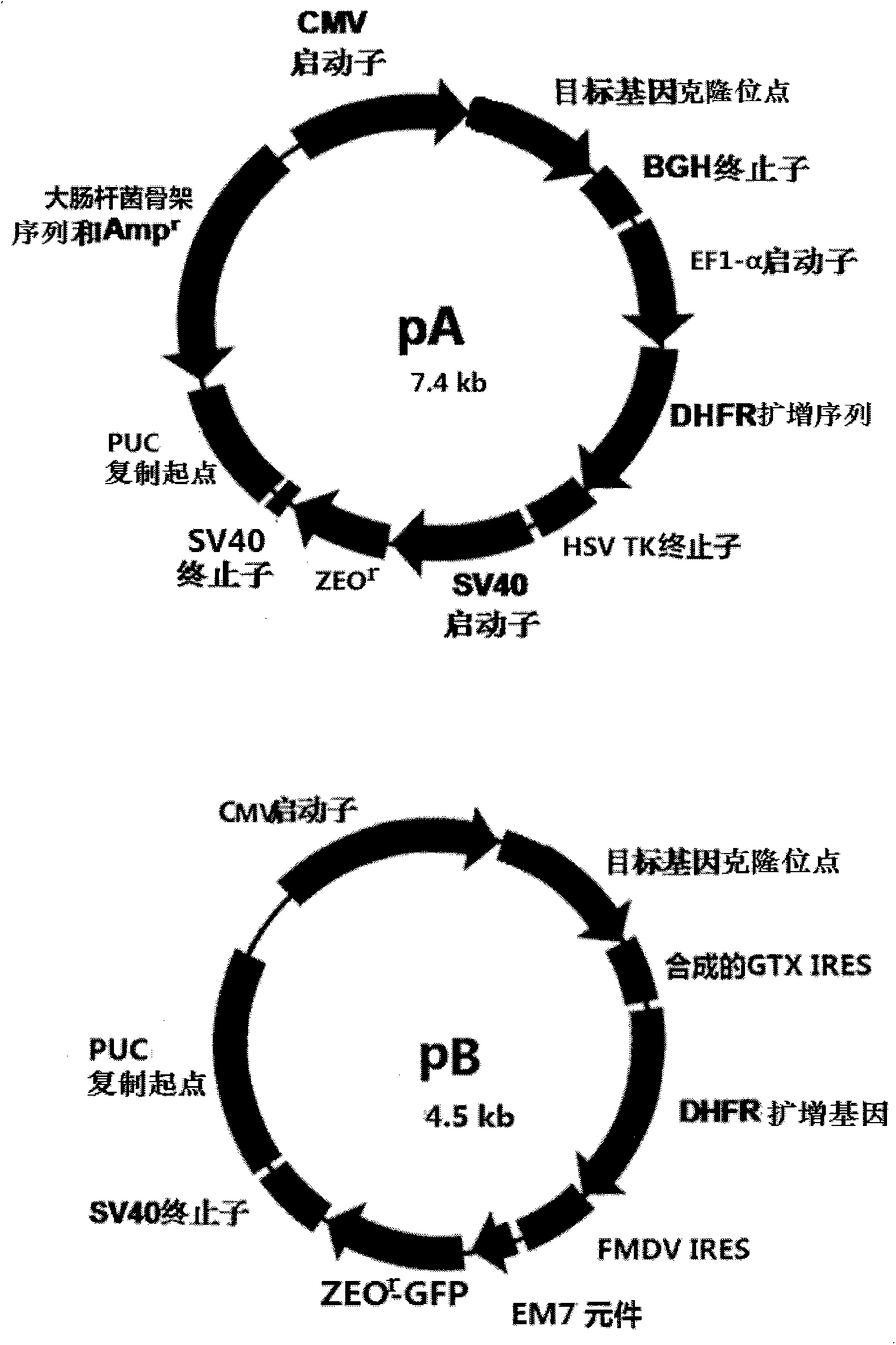
[0047] Embodiment 1: Construction of mammalian cell expression vector
[0048] Methods: The traditional expression vector pA was constructed by molecular biology techniques, such as restriction enzyme digestion, DNA ligation, Escherichia coli transformation and clone screening, according to the standard methods of "Molecular Cloning Experiment Guide" (third edition, translated by Huang Peitang, Science Press) and the expression vector pB of the present invention. The specific operation method is as follows:
[0049] 1.1 Construction of the traditional expression vector pA: the pcDNA3.1 vector of Invitrogen Company was used as the template.
[0050] The fusion sequence (1.8kb) of promoter SV40, DHFR, terminator sv40 and other regulatory sequences was artificially synthesized, and speI and NaeI enzyme cutting sites were added at its ends, respectively. The synthetic fusion sequence was ligated to pcDNA3.1 digested by speI and NaeI using T4DNA ligase, transformed into Escherich...
Embodiment 2
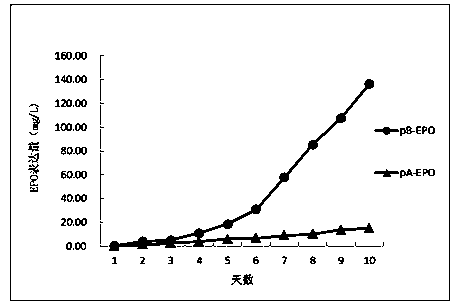
[0077] Example 2: Rapid screening of stable cell lines expressing EPO protein
[0078] 2.1. Construct the expression vector containing the target gene: artificially synthesize the coding sequence of EPO according to the existing sequence information of NCBI, and clone it into the expression vector pA constructed in Example 1 by introducing the corresponding restriction sites (NheI and HindIII) and pB, the expression plasmids pA-EPO and pB-EPO containing the target gene were obtained.
[0079] 2.2. DNA transfection into CHO-dhfr - Suspension cell lines: Transfect the above-mentioned expression plasmids pA-EPO and pB-EPO containing the target gene, and the expression vector control pA and pB into DHFR enzyme-deficient CHO cells (CHO-dhfr - )middle. CHO-dhfr - The cells were cultured in suspension, and the cell density was 1.5×10 6 / ml, the transfection volume was 30ml. The transfection method was operated according to the instruction of Lipofectamine2000 (purchased from Inv...
Embodiment 3
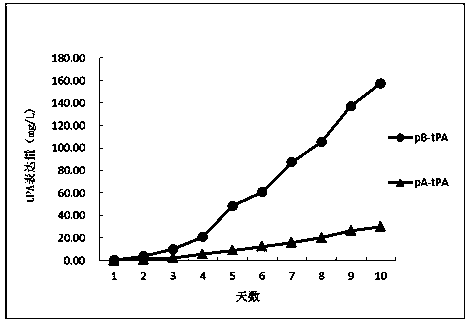
[0097] Embodiment 3: the comparison of EPO protein expression level in different host cells
[0098] Method: The expression vectors pA and pB constructed in Example 1 and the expression plasmids pA-EPO and pB-EPO containing the target gene EPO constructed in Example 2 were transfected into the following three host cells, including CHO-dhfr cells, BHK cells and HEK293 cells. The transfection method and the screening method for obtaining stable cell lines are the same as those in Example 2.
[0099] Results: Through Western Blotting and ELISA analysis, the expression of the target protein was not detected in the negative control (pA, pB) transfected cells, so the stable cell line containing EPO could not be screened. Compared with the negative control, the host cells transfected with expression plasmids pA-EPO and pB-EPO containing the target gene EPO obtained six kinds of stable cell lines through screening, and the ELISA analysis results showed that in the traditional screeni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com