Preparation method of guinea pig airway allergic asthma model
A technology for allergic asthma and guinea pigs, applied in the field of biomedicine, to achieve good repeatability, good sensitization effect, and strong pertinence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of Guinea Pig Airway Allergic Asthma Model
[0021] Experimental animals: 12 guinea pigs, half male and half male. Hartley-Dunkin, SPF grade, weighing 300-360 g, was purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[0022] The experimental ovalbumin (OVA) was purchased from SIGMA.
[0023] The breeding environment is as follows: temperature: 20-26°C; humidity: 40%-70%; ventilation rate: not less than 15 ventilation times per hour, using 100% fresh air (no air circulation). Lighting: Automatic lighting, alternating light and dark every 12 hours, turn off the lights at 7:00PM, and turn on the lights at 7:00AM the next day.
[0024] The guinea pig airway allergic asthma model preparation method is mainly divided into two stages: the sensitization period and the challenge period.
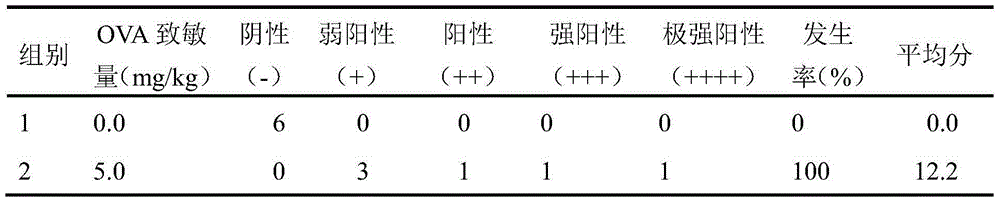
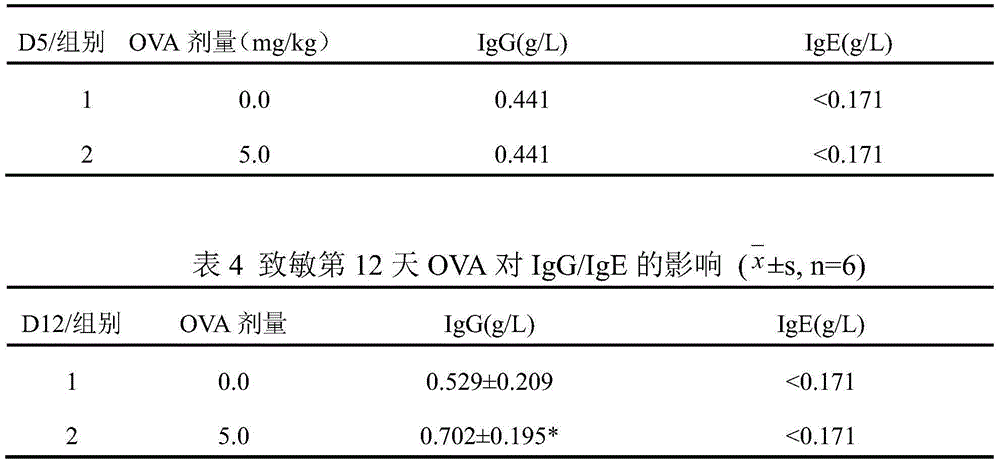
[0025] The sensitization period was: guinea pigs were sensitized by intraperitoneal injection of 3 mg / ml OVA normal saline solution 3 ml / kg,...
Embodiment 2
[0044] Example 2 Preparation of Guinea Pig Airway Allergic Asthma Model
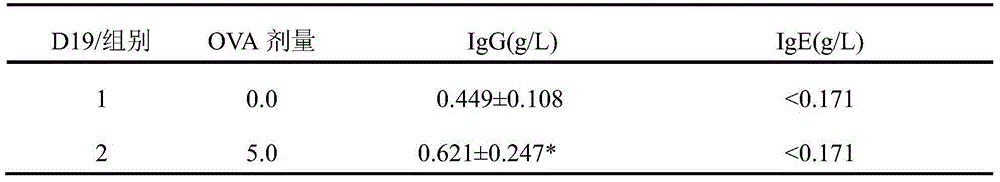
[0045] Please refer to the technical content described in Example 1 for the preparation method and feeding environment of the experimental animals. The guinea pig airway allergic asthma model preparation method is mainly divided into two stages: the sensitization period and the challenge period. The sensitization period was as follows: guinea pigs were sensitized by intraperitoneal injection of 2 mg / ml OVA normal saline solution 2 ml / kg, once every other day, and the administration time was D1, D3, D5 and D7 days, a total of 4 administrations. The challenge period is: 12 days after the last sensitization (D19), challenge with a single nebulized administration. Put the guinea pig into the animal test tube of the IN-TOX small animal nasal exposure system, and insert the animal test tube into the exposure chamber. Inhale the atomized OVA physiological saline solution (its concentration is 4mg / ml) to attac...
Embodiment 3
[0046] Example 3 Preparation of Guinea Pig Airway Allergic Asthma Model
[0047] Please refer to the technical content described in Example 1 for the preparation method and feeding environment of the experimental animals. The guinea pig airway allergic asthma model preparation method is mainly divided into two stages: the sensitization period and the challenge period. The sensitization period was: guinea pigs were sensitized by intraperitoneal injection of 5 mg / ml OVA saline solution 5 ml / kg, once every other day, and the administration time was D1 and D3, and the administration was administered twice in total. The challenge period is: 15 days after the last sensitization (D18), challenge with a single nebulized administration. Put the guinea pig into the animal test tube of the IN-TOX small animal nasal exposure system, and insert the animal test tube into the exposure chamber. Inhale the atomized OVA physiological saline solution (its concentration is 7mg / ml) to attack, se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com