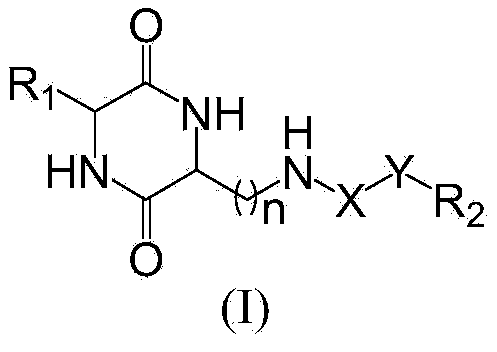
Piperazinedione derivative and preparation and application thereof
A compound and unsubstituted technology, applied in the field of piperazine diketone derivatives and its preparation, can solve problems such as increased use of pesticides, increased cost of pest control, environmental and ecological damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
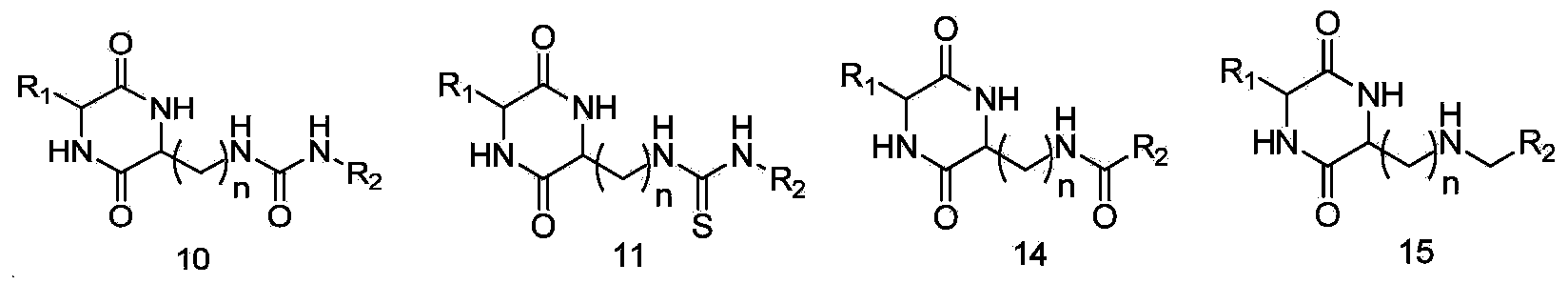
Image
Examples
preparation example Construction
[0056] A particularly preferred preparation process is as follows:
[0057]
[0058] (5) Dissolve triphosgene (BTC) in an inert solvent (such as dichloromethane, 1,2-dichloroethane, chloroform, carbon tetrachloride, DMF, etc.), and compound 6 and an organic base are dissolved in an inert solvent ( (such as dichloromethane, 1,2-dichloroethane, chloroform, carbon tetrachloride, etc.), add triphosgene in an inert solvent (such as dichloromethane) solution at low temperature to obtain compound 7.
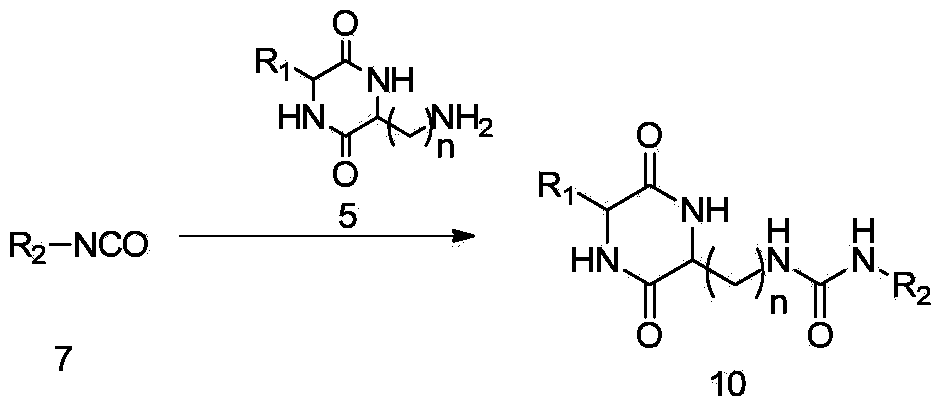
[0059] (6) Drop compound 7 into a solution of compound 5 and an organic base in an inert solvent (such as dichloromethane, 1,2-dichloroethane, chloroform, carbon tetrachloride, DMF, etc.) to obtain compound 10.
[0060] (7) Dissolving compound 6, carbon disulfide, and an organic base into an inert solvent (such as THF, toluene, methylene chloride, 1,2-dichloroethane, chloroform, carbon tetrachloride, etc.) solution to obtain compound 8 , adding triphosgene to give compound 9.
[00...
Embodiment 1
[0096] Embodiment 1: the synthesis of piperazine diketone intermediate 5a
[0097]
[0098] (1) Take ornithine hydrochloride 1a (337mg, 2mmol), di-tert-butyl dicarbonate ((Boc) 2 O) (1310mg, 6mmol), triethylamine (Et 3 N) (404mg, 4mmol) was dissolved in 6ml of acetone and 6ml of water, stirred at room temperature, followed by TCL. After the reaction, the acetone was distilled off under reduced pressure, and extracted three times with dichloromethane. The organic phase was washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation under reduced pressure to obtain the crude product of compound 2a.
[0099] (2) In a 100ml round bottom flask, add the crude product of compound 2a obtained in the first step reaction, tryptophan methyl ester hydrochloride (1019mg, 4mmol), then add triethylamine (6mmol), HOBt (297mg, 2.2mmol), EDCl (460mg, 2.4mmol), add 30ml of dichloromethane, stir in ice bath for 1 hour, and then stir...
Embodiment 2
[0101] Embodiment 2: the synthesis of piperazine diketone urea compound 10a-1
[0102]
[0103] Into a 50ml round bottom flask was added triphosgene (BTC) (223mg, 0.75mmol), dissolved in 5ml of dichloromethane. Then aniline 6a (140mg, 1.5mmol) and triethylamine (152mg, 1.5mmol) were dissolved in 5ml of dichloromethane, added dropwise to the previous flask, stirred in an ice bath, dripped for about half an hour, and stirred at room temperature (TLC tracking reaction) to obtain compound 7a. Take another 50ml round bottom flask, dissolve compound 5a (300mg, 1mmol) in 1ml DMF, add triethylamine (101mg, 1mmol), and stir magnetically. Slowly drop the previous reaction into the flask and react at room temperature. After the dropwise addition, the reaction was complete (TLC followed the reaction). After removing the solvent in the reaction system by distillation under reduced pressure, and washing with water, the obtained crude product was separated and purified by column chroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com