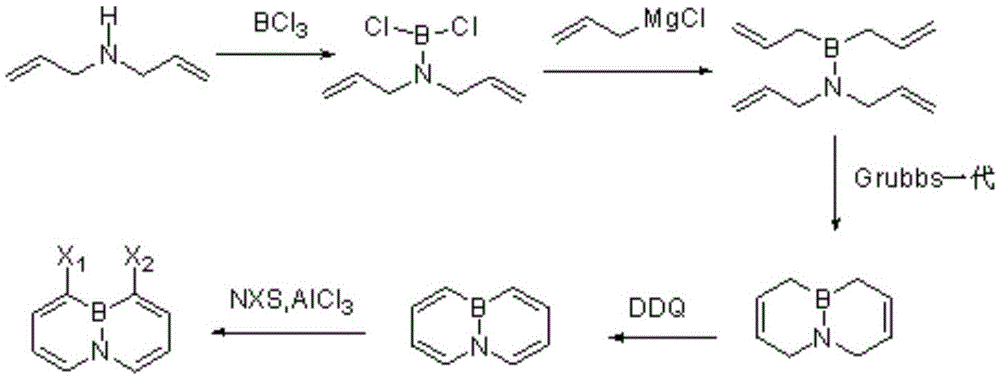
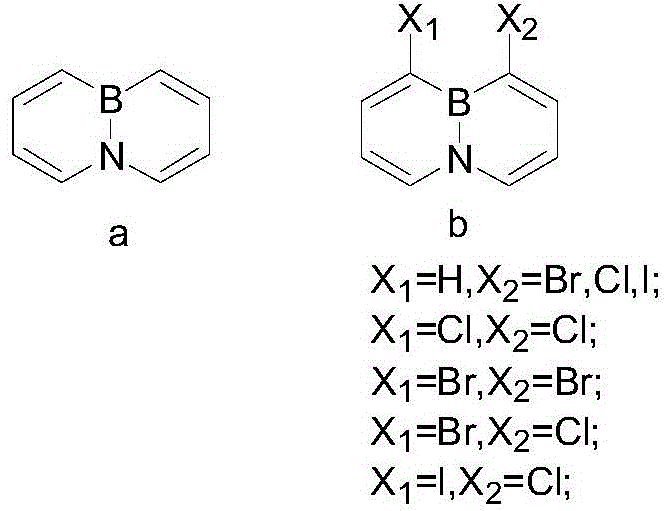A method for synthesizing 4a, 8a-borazyridine and derivatives thereof
A technology of derivatives and heteronaphthalene, which is applied in the field of synthesizing 4a,8a-borazanaphthalene and its derivatives, can solve the problems of inability to effectively prepare large quantities of target products, limit research on the properties of heteronaphthalene compounds, and low overall yields , to achieve great scientific research value and commercial application prospects, simple and efficient post-processing process, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] synthesis
[0036] Take a 500mL Schlenk bottle, under a nitrogen atmosphere, at -35°C, add diallylamine (25.2g, 520mmol) dropwise to boron trichloride (16.4g, 140mmol) in dichloromethane as a solvent, a total of 200mL, at room temperature Stir overnight. Atmospheric pressure distills out dichloromethane, pentane extracts the residue, normal pressure steams off pentane, and then vacuum distillation obtains the product diallylamine boron dichloride as (10.4g, 45%) colorless flammable liquid , boiling point 25°C (1Torr). 1 HNMR (400MHz, C 6 D. 6 ): δ5.42-5.32 (m, 2H), 4.88 (d, J = 7, 6Hz, 2H), 4.84 (d, J = 17, 6Hz, 2H), 3.53 (d, J = 4.7Hz, 4H) . 13 CNMR (100Hz, C 6 D. 6 ): δ133.9, 117.1, 51.6. 11 BNMR (128MHz, C 6 D. 6 ): δ31.5.
Embodiment 2
[0038] synthesis
[0039]Take 20g of magnesium chips, 5mL of allyl chloride, a few grains of elemental iodine, and 60mL of tetrahydrofuran as a solvent and place them in a three-necked flask. After triggering, the remaining allyl chloride in tetrahydrofuran solution is about 180mL. The prepared allylmagnesium chloride is the Grignard reagent with a concentration of 0.8-1.2 mol / L. Take 18g of diallylamino boron dichloride and 100ml of tetrahydrofuran as a solvent in the glove box, add 200mL of Grignard reagent with a concentration of 1.05mol / L with an injection needle at low temperature, and stir overnight at room temperature. First distill off most of THF, then add hexane for multiple extractions, discard the white precipitate, transfer the extract to another flask, first distill off the solvent hexane, then distill under reduced pressure to obtain (13.8g, 70%) colorless Liquid, boiling point 50°C (0.133KPa). 1 HNMR (400MHz,C 6 D. 6 ):δ6.02-5.92(m,2H),5.59-5.50(m,2H),5.0...
Embodiment 3
[0041] synthesis
[0042] Take diallylaminodiallyl borane (8.20 g, 43 mmol) from the glove box, add 80 mL of dichloromethane, and put it in a -35°C environment. Take (0.28g, 0.34mmol, 0.8% catalytic amount) first-generation Grubbs catalyst and add 40mL of dichloromethane to dissolve it. The catalyst was introduced into the reaction bottle, and the reaction was continued at low temperature for 1 hour, then slowly raised to room temperature, and stirred overnight. The dichloromethane was distilled off under normal pressure, and distilled under reduced pressure to obtain (5.13 g, 90%), a colorless and easily crystalline liquid with a boiling point of 20°C (1 Torr). 1 HNMR (400MHz,C 6 D. 6 ): δ5.48, (d, J=10Hz, 2H), 3.15(m, 4H), 1.42(d, J=4.6Hz, 4H). 13 CNMR (100Hz, C 6 D. 6 ): δ126.1, 124.5, 50.2, 16.6(bs). 11 BNMR (128MHz, CDCl 3 ):δ41.3.HRMS(ESI-TOF)m / z calcd.For C 8 h 13 BN[M+H]: 134.1137, found: 134.1163.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com