Method for Determination of Related Substances of Ebastine by High Performance Liquid Chromatography
A high-performance liquid chromatography and ebastine technology, which is applied in the field of high-performance liquid chromatography for the determination of ebastine related substances, can solve the problems of tailing, unfavorable popularization and application, inconvenient measurement, and saves detection time. , The effect of high detection accuracy and shortened cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Chromatographic conditions: mobile phase A is phosphate buffer (take 3.58g of disodium hydrogen phosphate dodecahydrate, add water to dissolve and dilute to 1000ml, adjust the pH value to 6.3 with phosphoric acid, add 1.92g of sodium lauryl sulfate, mix well )-acetonitrile=80:20, mobile phase B is acetonitrile; flow rate: 1.0ml / min; perform linear gradient elution according to Table 1.
[0077] Preparation of the test sample solution: take an appropriate amount of this product, add a solvent to dissolve and dilute to make a solution containing 2.0 mg of ebastine per 1 ml, as the test sample solution; accurately measure the test sample solution in an appropriate amount, dilute with a solvent to prepare A solution containing 2 μg of ebastine per 1 ml was used as a control solution. Inject the sample and record the chromatogram.
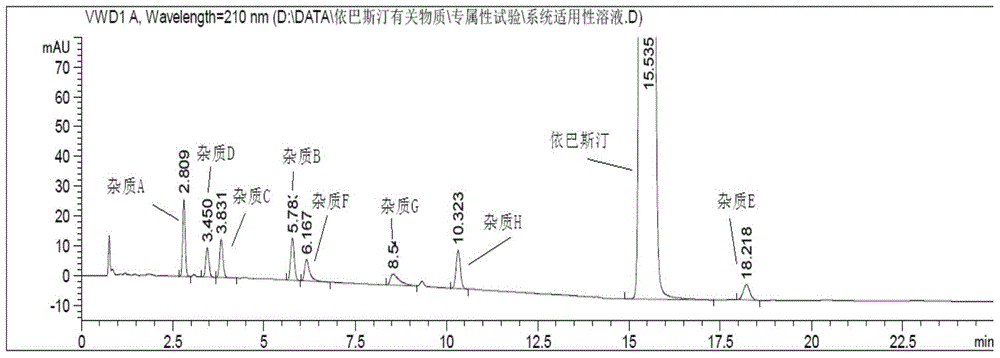
[0078] Test results such as figure 1 shown. figure 1 Among them, the 8 kinds of impurities in the ebastine raw material drug were completely ...
Embodiment 2-3
[0080] Chromatographic conditions: mobile phase A is phosphate buffer [take (Example 2-dipotassium hydrogen phosphate 2.28g), (Example 3-diammonium hydrogen phosphate 1.32g), add water to dissolve and dilute to 1000ml, adjust pH with phosphoric acid value to 6.3, add 1.92g of sodium lauryl sulfate, mix]-acetonitrile (80:20), mobile phase B is acetonitrile; flow rate: 1.0ml / min; linear gradient elution as listed in Table 1.
[0081] Preparation of the test solution: take an appropriate amount of this product, add solvent to dissolve and dilute to make a solution containing about 2.0 mg of ebastine per 1 ml, as the test solution; accurately measure the appropriate amount of the test solution, and dilute it with a solvent Prepare a solution containing about 2 μg of ebastine per 1 ml as a control solution. Inject the sample and record the chromatogram.
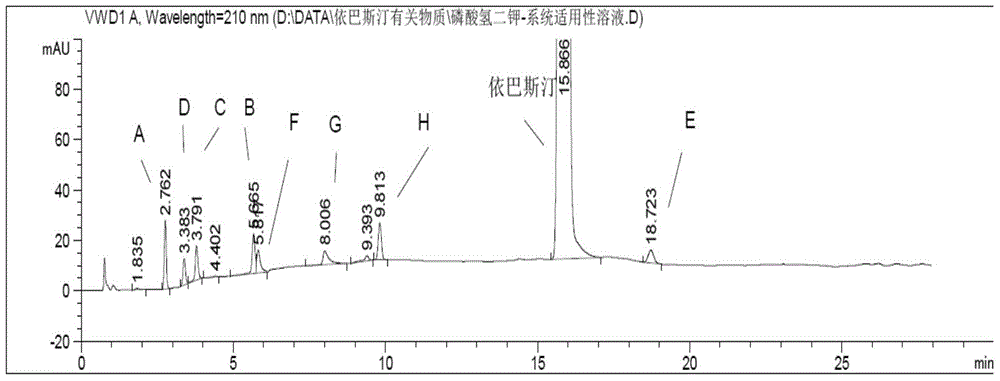
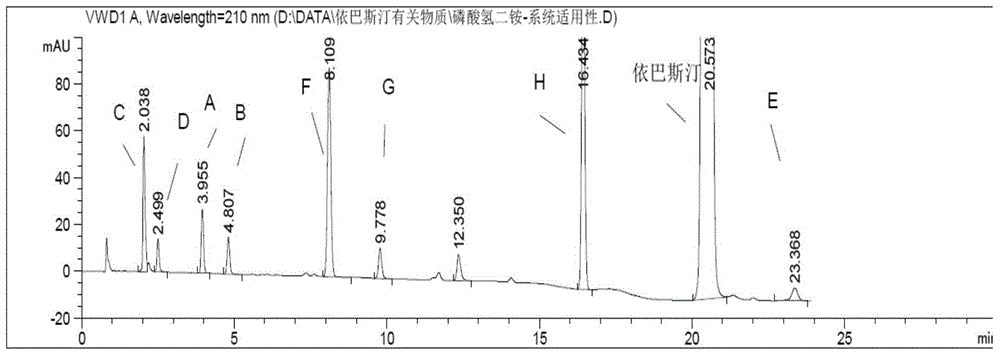
[0082] Embodiment 2 result sees attached figure 2 ; Embodiment 3 results are attached image 3 . pass figure 2 , image ...
Embodiment 4-5
[0084] Chromatographic conditions: mobile phase A is phosphate buffer (1.79g of disodium hydrogen phosphate dodecahydrate is taken in embodiment 4, 17.9g of disodium hydrogen phosphate dodecahydrate is taken in embodiment 5, dissolved in water and diluted to 1000ml, adjusted with phosphoric acid When the pH value reaches 6.3, add 1.92 g of sodium lauryl sulfate, mix well)-acetonitrile (80:20), mobile phase B is acetonitrile; flow rate: 1.0ml / min; perform linear gradient elution as listed in Table 1.
[0085] Preparation of the test solution: take an appropriate amount of this product, add solvent to dissolve and dilute to make a solution containing about 2.0 mg of ebastine per 1 ml, as the test solution; accurately measure the appropriate amount of the test solution, and dilute it with a solvent Prepare a solution containing about 2 μg of ebastine per 1 ml as a control solution. Inject the sample and record the chromatogram.
[0086] Embodiment 4 result sees attached Figure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com