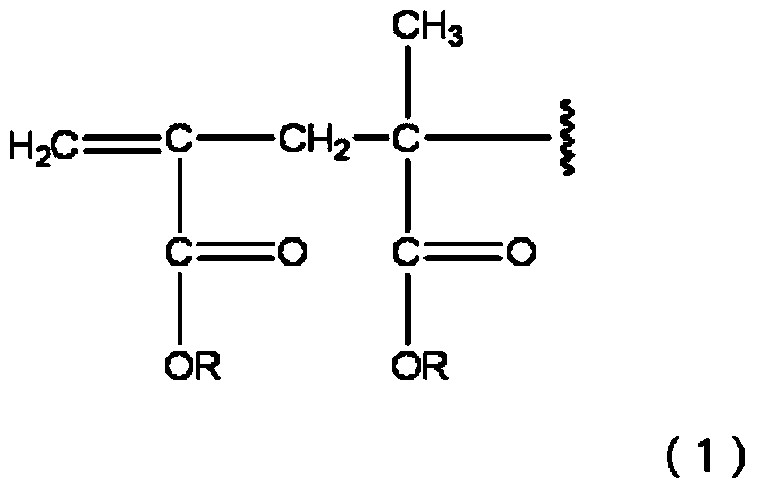
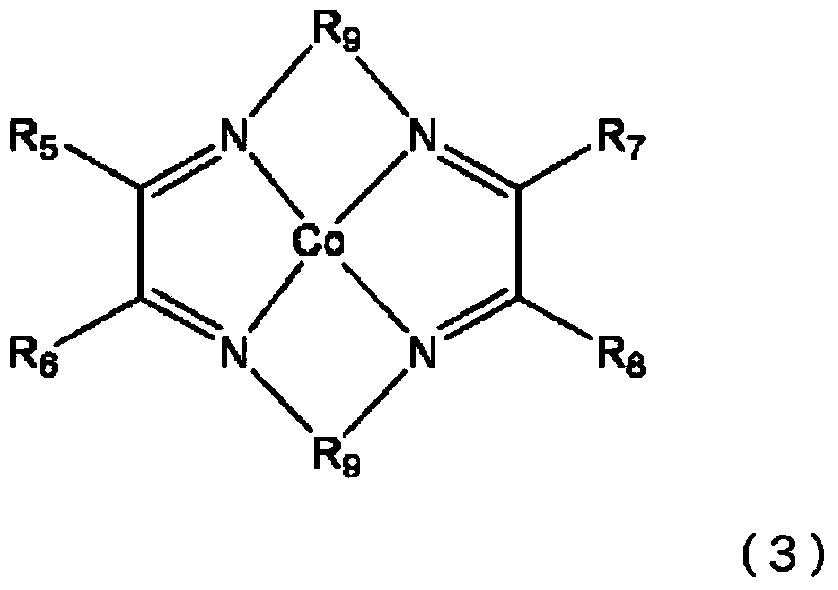
Methacrylic acid ester polymer, method for producing same, active energy ray-curable composition, and optical recording medium
A kind of methacrylate, the technology of the production method, applied in the field of active energy ray-curable resin composition, optical recording medium, to achieve the effect of hardness corrosion, high hardness, low warpage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0194] Hereinafter, although an Example demonstrates this invention more concretely, this invention is not limited to this. In addition, below, "part" means a "mass part". In addition, the measurement and evaluation of each physical property were performed by the following methods.
[0195]
[0196] The methacrylate polymer was dissolved in deuterated chloroform, and 1H-NMR measurement was performed using a nuclear magnetic resonance apparatus UNITY INOVA 500 superconducting FT-NMR (trade name) manufactured by Varian. Peaks derived from terminal double bonds were confirmed at 5.5 and 6.2 ppm, identifying the terminal structure.
[0197] The number average absolute molecular weight was measured by GPC-LALLS measurement using HLC-8220GPC manufactured by Tosoh Corporation and TriSEC302TDA manufactured by Viscotek Corporation. The number average degree of polymerization was obtained by dividing the number average absolute molecular weight by the average molecular weight of the...
manufacture example 1
[0226] [Manufacture Example 1] Manufacture of Dispersant 1
[0227] Add 900 parts of deionized water, 60 parts of 2-ethanesulfonate sodium methacrylate, 10 parts of potassium methacrylate and 12 parts of methyl methacrylate into a polymerization device equipped with a stirrer, a cooling pipe, and a thermometer, and stir. The temperature was raised to 50° C. while substituting nitrogen in the polymerization apparatus. 0.08 part of 2,2'- azobis(2-methylpropionamidine) dihydrochlorides were added here as a polymerization initiator, and it heated up to 60 degreeC further. After the temperature was raised, methyl methacrylate was continuously dripped at a rate of 0.24 parts / minute for 75 minutes using a drip pump. After maintaining the reaction solution at 60° C. for 6 hours, it was cooled to room temperature to obtain a dispersant 1 having a solid content of 10% by mass as a transparent aqueous solution.
manufacture example 2
[0228] [Production Example 2] Production of chain transfer agent 1 (transition metal chelate)
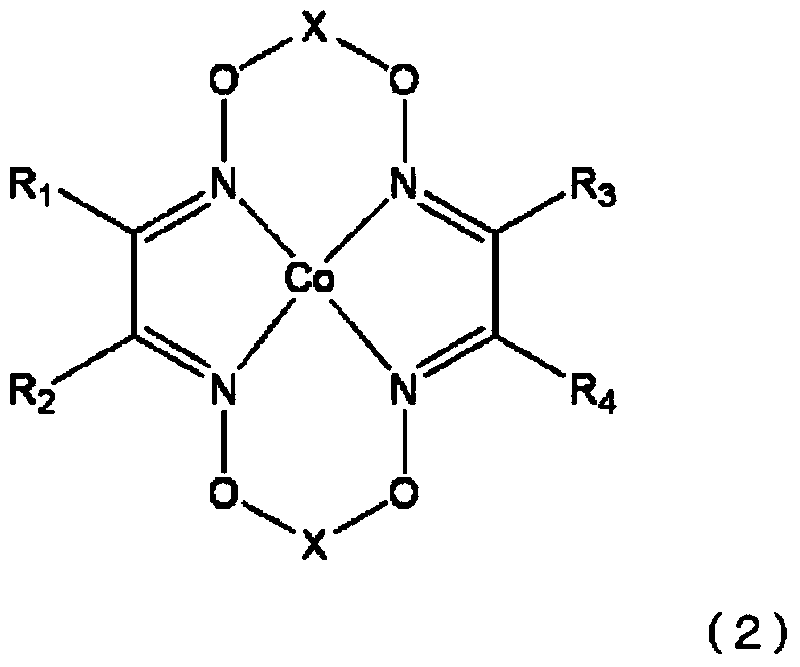
[0229] In a synthesis device equipped with a stirring device, cobalt(II) acetate tetrahydrate (Co(OC(=O)CH 3 ) 2 4H 2 O) 2.00 g (8.03 mmol), 3.86 g (16.1 mmol) of benzophenone oxime, and 100 ml of diethyl ether previously deoxygenated by nitrogen bubbling were stirred at room temperature for 2 hours. Next, 20 ml of boron trifluoride diethyl ether complex was added, followed by further stirring for 6 hours. The mixture was filtered, the solid was washed with diethyl ether, and dried for about 12 hours at 20°C and under vacuum conditions below 100Mpa to obtain chain transfer agent 1 (formula ( 1), R 1 ~R 4 is phenyl, X is BF 2 indicated compounds).
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com