Segmented polyester copolymer and preparation method thereof
A polyester block and copolymer technology, applied in the field of polymers, can solve the problems of low polyester molecular weight, poor chain extension efficiency, and imperfect preparation methods of polyester block copolymers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
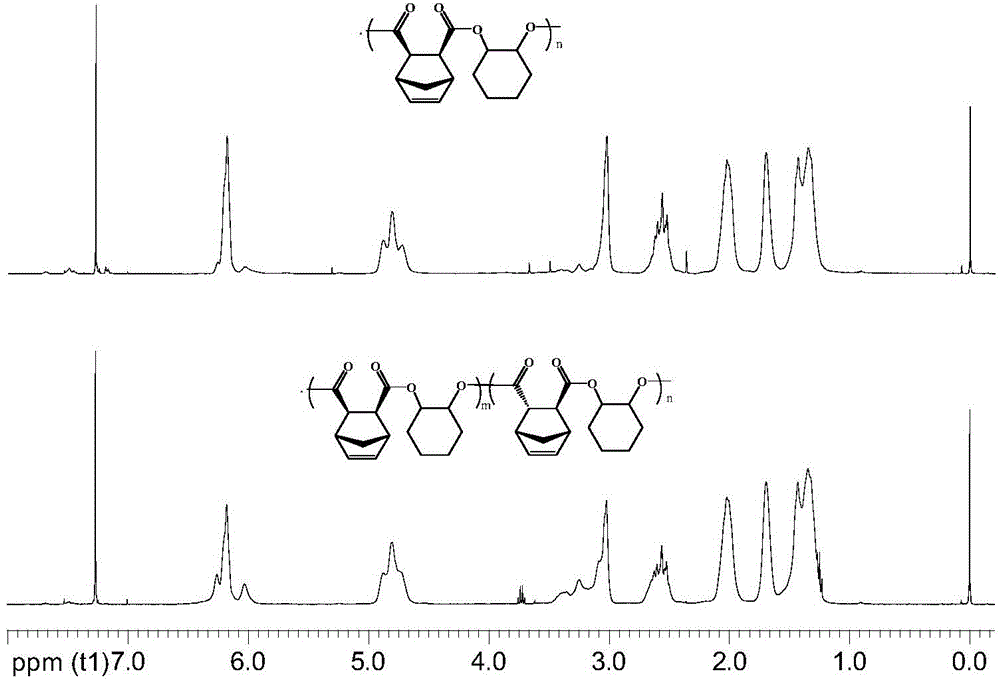
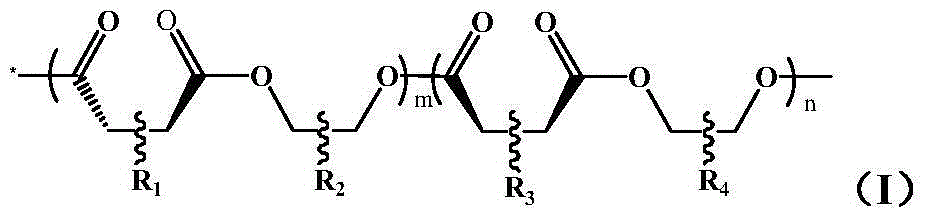
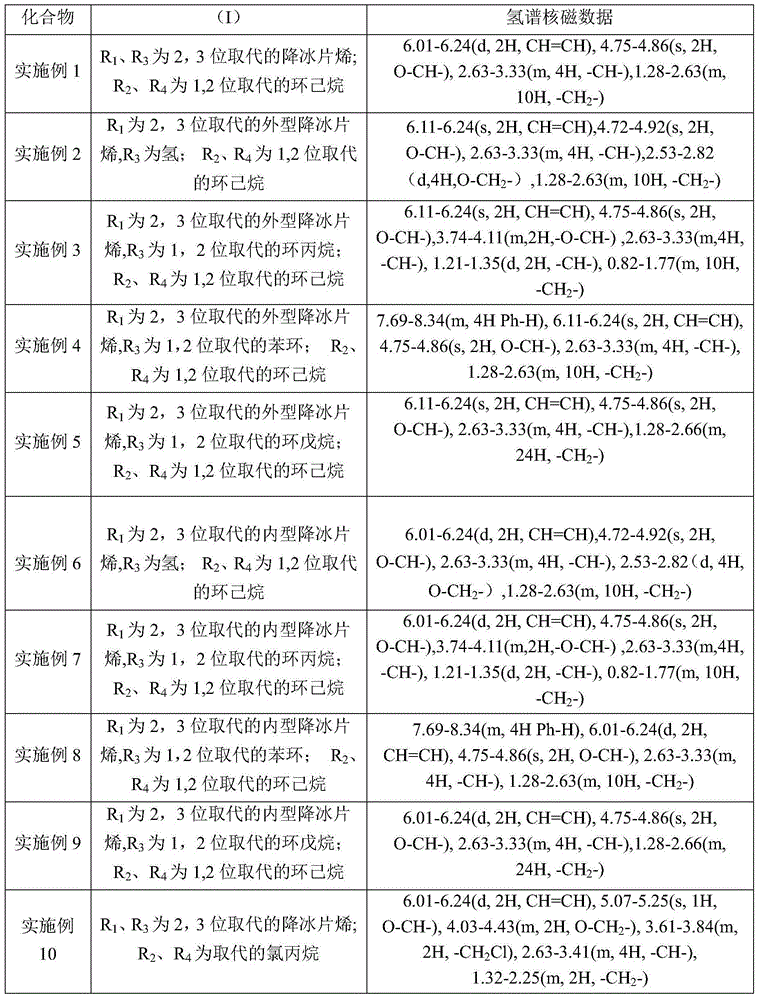
[0033] Take a dry and clean 25ml thick-walled pressure-resistant reaction tube under argon atmosphere, add 5mmol exo-norbornene dianhydride, 0.1mmol bis-(triphenylphosphoryl)ammonium chloride, 5mmol epoxycyclohexane, 1ml Dissolve in toluene (the amount of solvent can dissolve quaternary ammonium salt compounds, epoxy compounds, acid anhydrides, etc.), react at 110°C for 1 hour; then add 5 mmol of internal norbornene diacid anhydride, 5 mmol of epoxy ring A mixed solution of hexane and 1ml of toluene (the amount of toluene can dissolve quaternary ammonium compounds, epoxy compounds, acid anhydrides, etc.), after reacting at 110°C for 4 hours, pour the reaction system into methanol with a volume of 50ml to settle , filtered, washed, and the resulting product was vacuum-dried at 45°C for 24h to obtain R 1 , R 3 For 2,3 substituted norbornene; R 2 , R 4 It is a polyester block copolymer with 1,2 substituted cyclohexane.
[0034] Among them, the m value is 15, and the n value i...
Embodiment 2
[0037] All the other operations are the same as in Example 1, and the difference is that in the step a) the acid anhydride is 5mmol external norbornene dioic anhydride, and the epoxy compound is 5mmol epoxycyclohexane; in the step b), the acid anhydride is 5mmol succinic anhydride, the epoxy compound It is 5mmol epoxycyclohexane. get R 1 It is the exo-norbornene substituted at the 2 and 3 positions, R 3 is hydrogen; R 2 , R 4 It is a polyester block copolymer of 1,2-substituted cyclohexane. The yield is 84%, the average molecular weight measured by GPC is 7050, the m value is 13, the n value is 18, and the molecular weight distribution is 1.6.
Embodiment 3
[0039] All the other operations are the same as in Example 1, and the difference is that in the step a) the acid anhydride is 5mmol external norbornene dioic anhydride, and the epoxy compound is 5mmol epoxycyclohexane; in the step b), the acid anhydride is 5mmol 3-oxabicyclo [3.1.0] The hexane-2,4-dione and the epoxy compound are 5 mmol of cyclohexane oxide. get R 1 It is the exo-norbornene substituted at the 2 and 3 positions, R 3 For 1,2-substituted cyclopropane; R 2 , R 4 It is a polyester block copolymer of 1,2-substituted cyclohexane. The yield is 85%, the average molecular weight measured by GPC is 5775, the m value is 11, the n value is 14, and the molecular weight distribution is 1.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com