Pharmaceutical composition for removing chloasma
A technology for removing chloasma and a composition, which is applied in the directions of drug combination, pharmaceutical formula, plant raw materials, etc., can solve the problems of difficult symptomatic treatment, repeated raw materials, etc., and achieve the effects of obvious effect, good inhibitory effect, and good synergistic elimination effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
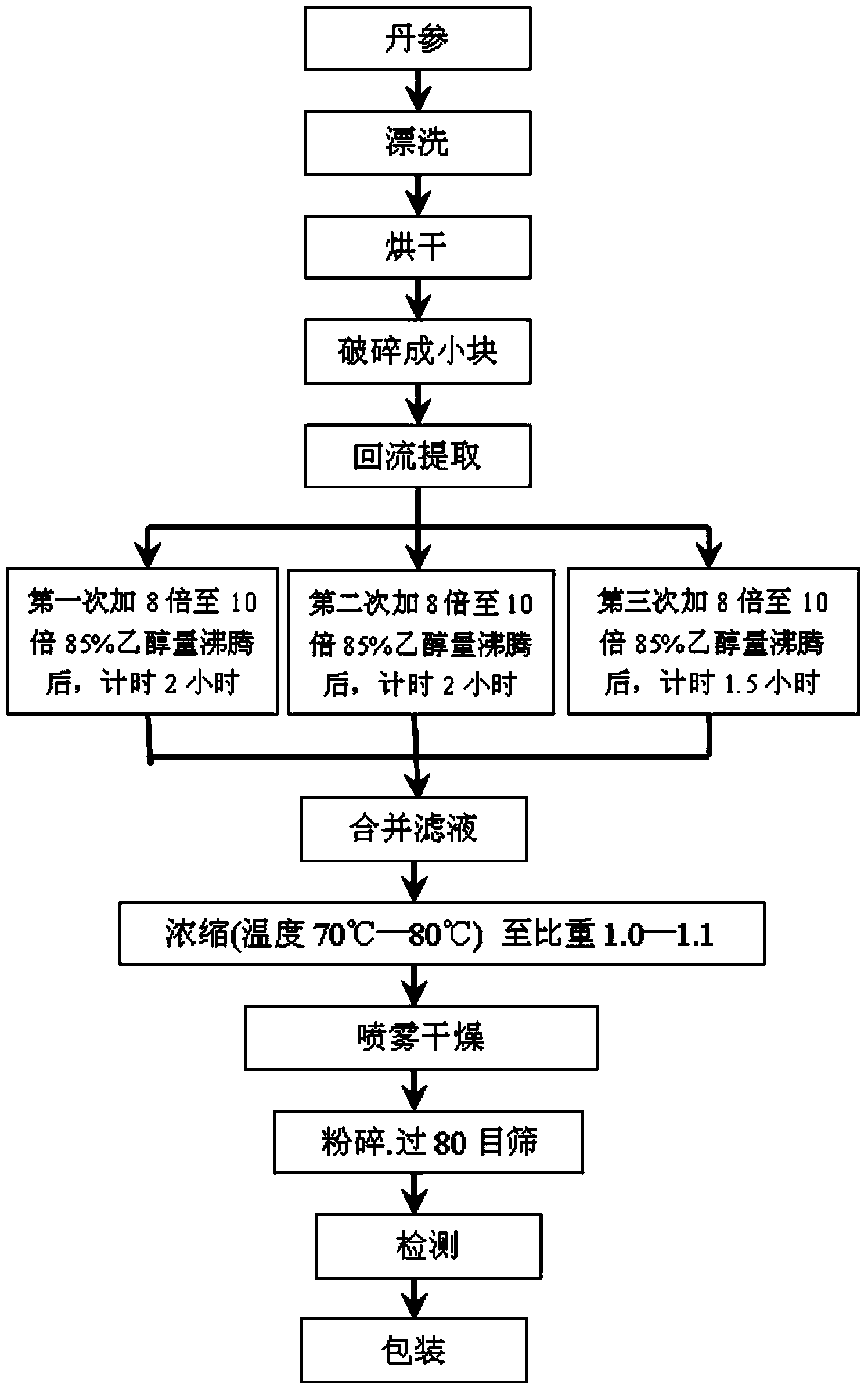
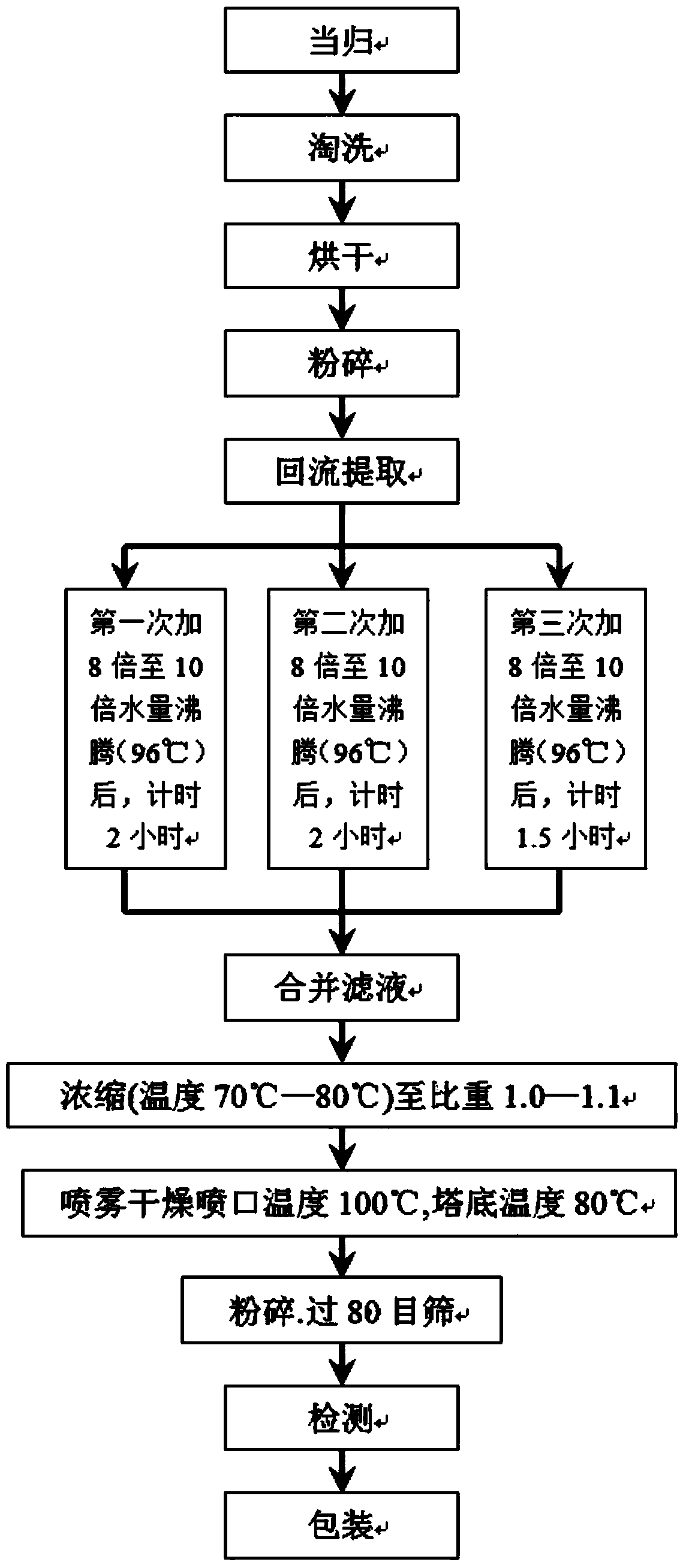
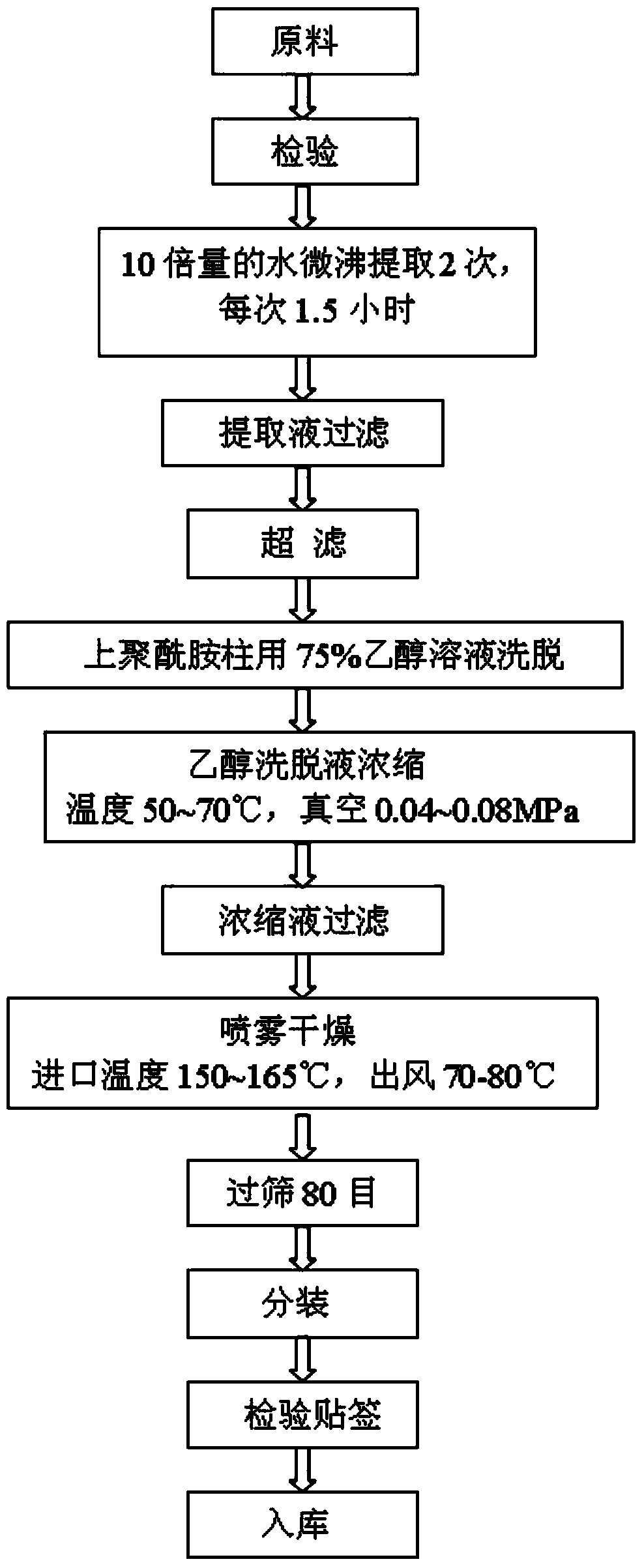
[0137] refer to Figure 1 to Figure 5 , Preferred Embodiment 1 of the present invention provides a pharmaceutical composition for removing chloasma, which contains the following raw material components in percentage by weight: Salvia miltiorrhiza extract 20-60%, Angelica sinensis extract 20-40%, Green tea extract tea polyphenols 10-30%, rose extract 10-35%, grape seed extract 5-30%.
[0138] The preparation method of the salvia miltiorrhiza extract: wash and dry the medicinal material salvia miltiorrhiza, crush it into small pieces, weigh the crushed salvia miltiorrhiza, put it into an extraction tank, add ethanol with a content of 85%, and the amount of ethanol added is the weight of the salvia miltiorrhiza 8-10 times (W / W), and extracted three times with ethanol under reflux. During the first reflux extraction, start timing from heating to boiling 96°C, and reflux extraction for 2 hours; after the first reflux extraction is completed, the second operation is the same as abo...
Embodiment 2
[0144] Formula (per 1000 capsules): 130g of Danshen extract, 135g of angelica extract, 50g of tea polyphenols, 80g of rose flower extract, 90g of grape seed extract, 10g of dextrin, and 5g of magnesium stearate. The raw materials are sieved, mixed evenly, and then supplementary materials magnesium stearate and dextrin are added appropriately, mixed evenly, poured into capsules to prepare capsules.
Embodiment 3
[0146] Formula (calculated per 1000 tablets): 128g of Danshen extract, 122g of angelica extract, 60g of tea polyphenols, 90g of rose flower extract, 80g of grape seed extract, 15g of dextrin, and 5g of magnesium stearate. The raw materials are sieved, mixed evenly, and then supplementary materials magnesium stearate and dextrin are added, mixed evenly, and compressed into tablets to obtain tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com