Functionalized ionic liquid used for modifying lipase, preparing method and the lipase obtained by modification
An ionic liquid, lipase technology, applied in biochemical equipment and methods, enzymes, hydrolase and other directions, can solve the problems of cumbersome product processing, lack of active functional groups in molecular structure, limited modification effect, etc., to achieve good biocompatibility The effect of stability and reactivity, saving activation time and cost, and simple and flexible method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
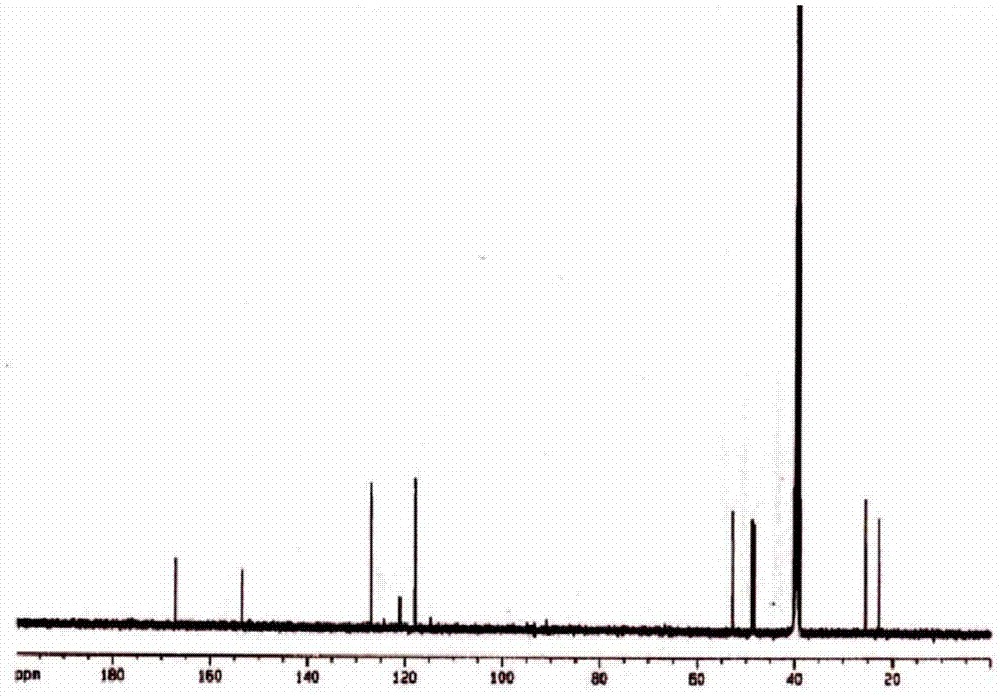
[0042] 1) Synthesis of 6,7-dihydro-5H-pyrrole[1,2-α]-3-carboxyethylimidazolium bromide: 0.203g6,7-dihydro-5H-pyrrole[1,2-α ] imidazole (CAS: 59646-16-1) and 2 mL of dichloromethane (DCM) were mixed evenly, then 0.374 g of methyl bromoacetate was added, and heated to reflux for 8 h. After the reaction was finished, 3M dilute hydrochloric acid was slowly added dropwise to adjust the pH to 2, the organic phase was collected, the solvent was removed under reduced pressure, and the column was separated with a yield of 91%.
[0043] 2) Activation of functionalized ionic liquids: 0.30g of 6,7-dihydro-5H-pyrrole[1,2-α]-3-carboxyethylimidazolium bromide and 0.2g of CDI were dissolved in 5mL of DMSO, at room temperature The reaction was carried out for 2 hours to obtain the activated functionalized ionic liquid, which was untreated and refrigerated at 4°C for later use.
[0044] 3) Covalent modification of enzymes: After activation, the functionalized ionic liquid was mixed with free T...
Embodiment 2
[0050] 1) Synthesis of 6,7-dihydro-5H-pyrrole[1,2-α]-3-carboxyethylimidazolium bromide: 0.203g6,7-dihydro-5H-pyrrole[1,2-α ] imidazole and 2mL DCM were mixed uniformly, then 0.374g methyl bromoacetate was added, and the reaction was heated under reflux for 8h with a yield of 91%.
[0051] 2) Activation of functionalized ionic liquids: 0.3g of 6,7-dihydro-5H-pyrrole[1,2-α]-3-carboxyethylimidazolium bromide, 0.2g of EDC·HCl and 0.12g of NHS were dissolved in 10ml of MES was reacted at 30°C for 1h.
[0052] 3) Enzyme modification: The activated functionalized ionic liquid and Thermomyces lanuginosus lipase were mixed and reacted at a molar ratio of 200:1, the reaction temperature was 0-4°C, the reaction time was 8 hours, and concentrated by ultrafiltration and centrifugation enzyme.
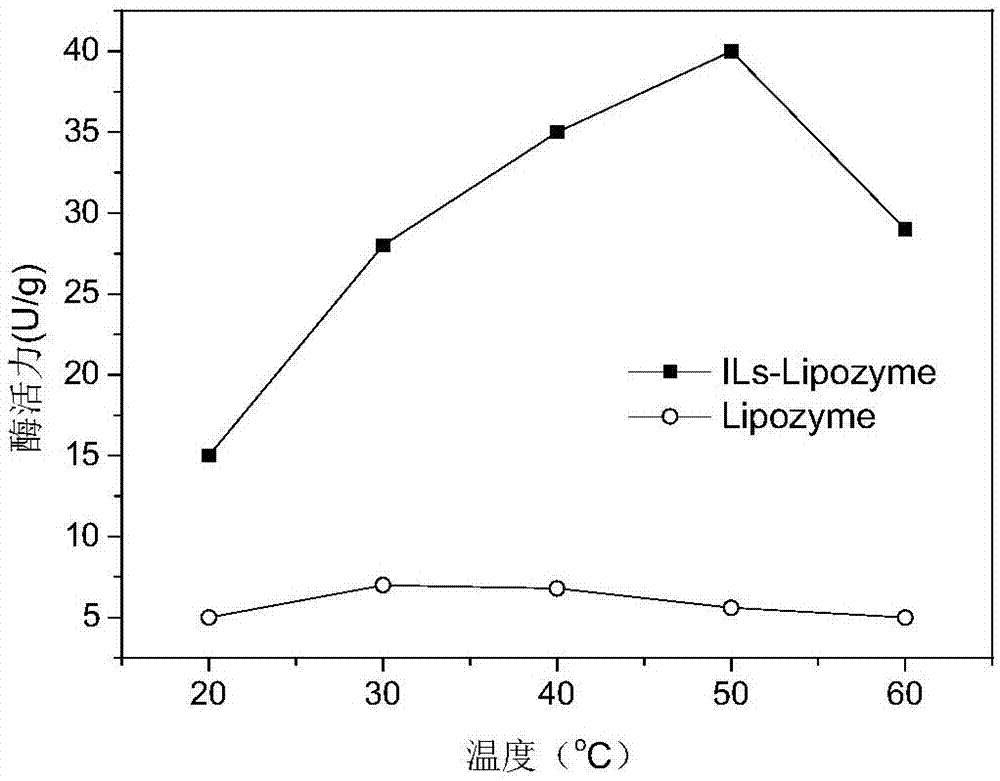
[0053] 4) Same as the enzyme activity assay conditions in Example 1, the transesterification activity of the covalently modified Thermomyces lanuginosus lipase (ILs-Lipozyme) measured at 30°C was ...
Embodiment 3
[0055] 1) Synthesis of 5,6,7,8-tetrahydropyridine[1,2-α]-3-carboxyethylimidazolium bromide: 0.215g of 5,6,7,8-tetrahydropyridine[1,2 -α]imidazole (CAS: 34167-66-3) was mixed with 2 mL of DCM evenly, then 0.374 g of methyl bromoacetate was added, and heated to reflux for 8 hours, with a yield of 90.3%.
[0056] 2) Activation of functionalized ionic liquid: Dissolve 0.31g of 5,6,7,8-tetrahydropyridine[1,2-α]-3-carboxyethylimidazolium bromide, 0.28g of EDC·HCl and 0.115g of NHS in In 10ml MES, react at room temperature for 1.5h.
[0057] 3) Enzyme modification: The activated functionalized ionic liquid and Candida antarctica lipase were mixed and reacted at a molar ratio of 200:1, the reaction temperature was controlled at 0-4°C, the reaction time was 4 hours, and the excess modifier was removed by ultrafiltration and centrifugation , Enzyme concentration.
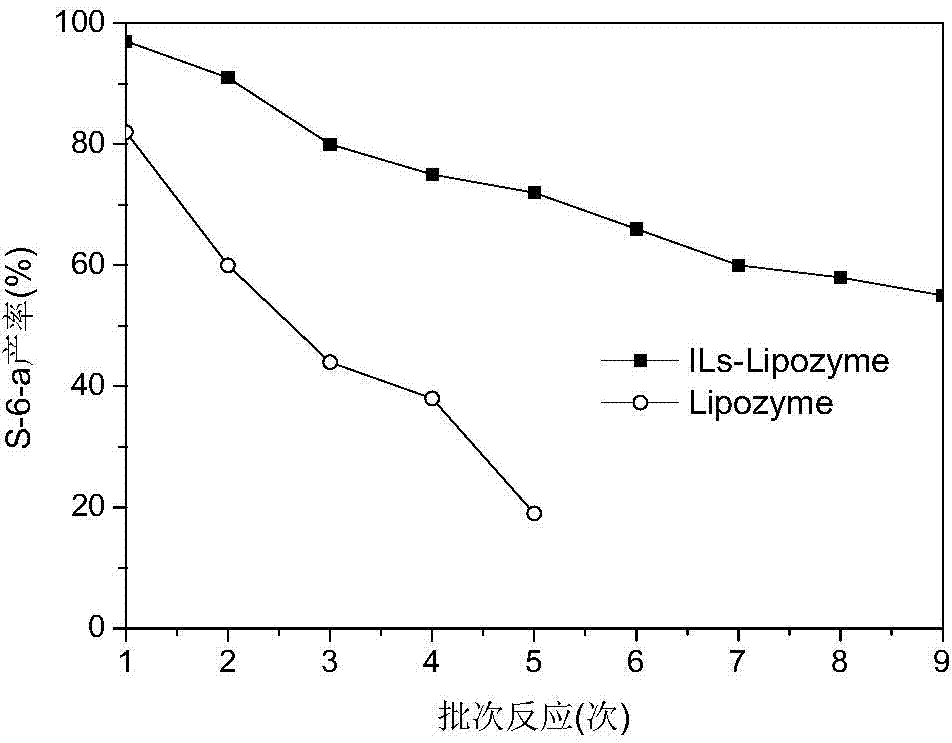
[0058] 4) Same as the enzyme activity determination conditions in Example 1, the transesterification activity of the cova...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com