Insoluble polysaccharide compound with hemostatic function and preparation method thereof
An insoluble and complex technology, applied in the field of biomedical materials, can solve the problems of high activity, low thrombin adsorption, limited application, etc., and achieve the effect of high activity and high coagulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
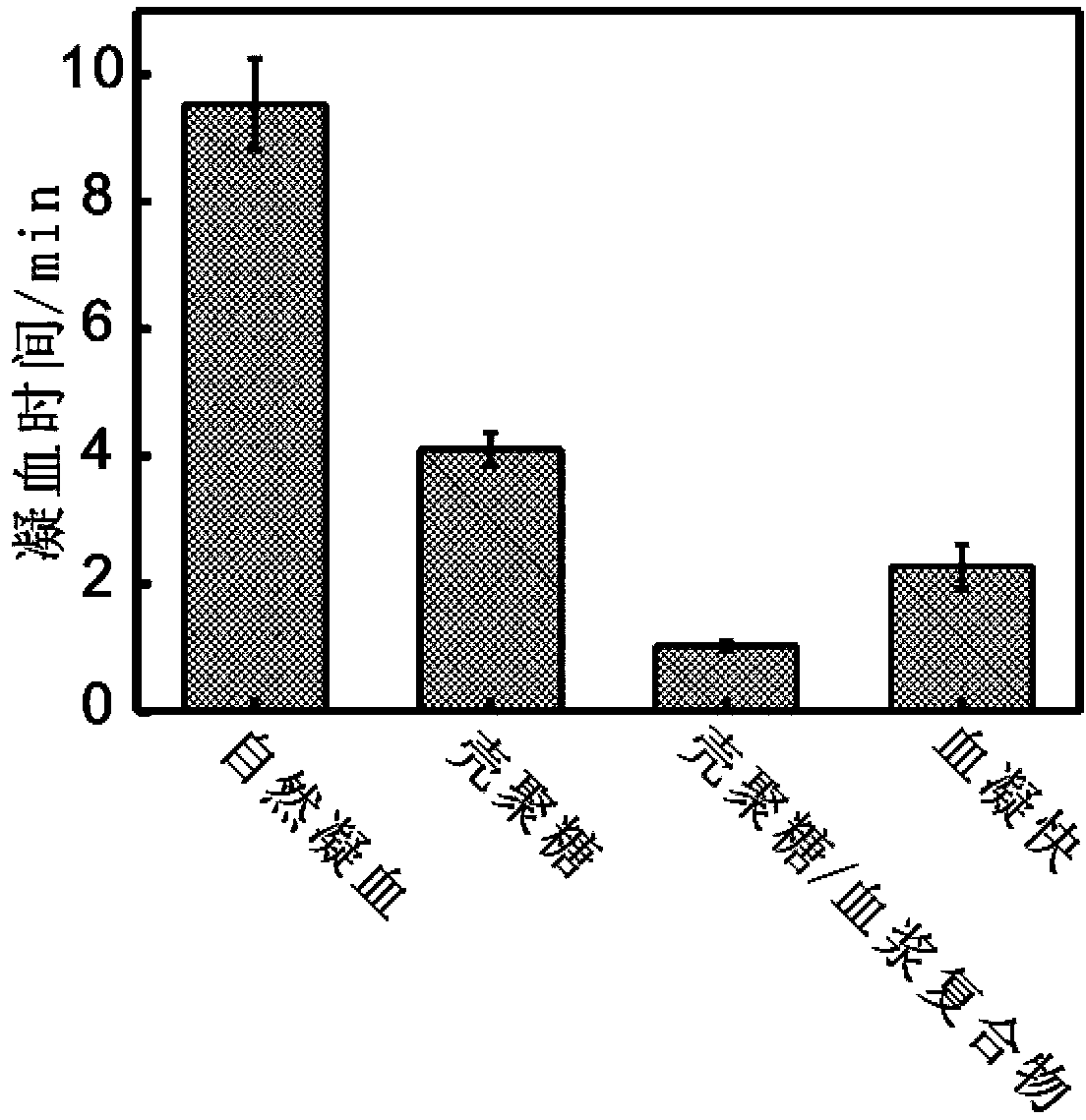
[0082] Preparation of chitosan / plasma complex: mix 50mg chitosan material (molecular weight 150,000-200,000) with 100ul anticoagulated plasma (according to the mass ratio of 1:2), add calcium ion to make the concentration 5mM, 25 °C for half an hour to obtain a chitosan / plasma composite material, which was vacuum-dried at 30°C for 5 hours to obtain a chitosan / plasma composite.
[0083] Determination of the coagulation effect of the chitosan / plasma complex: grind the chitosan / plasma complex, and conduct an in vitro coagulation experiment at 25°C, and keep the whole blood in a water bath at 25°C for half an hour in advance. Take 50mg chitosan / plasma complex, then add 40ul of 0.2M CaCl 2 , finally add 2ml of constant temperature whole blood, investigate its coagulation effect, the time when the whole blood coagulates is recorded as the coagulation time of chitosan / plasma complex. Repeat the above-mentioned steps of measuring blood coagulation time three times, get the average va...
Embodiment 2
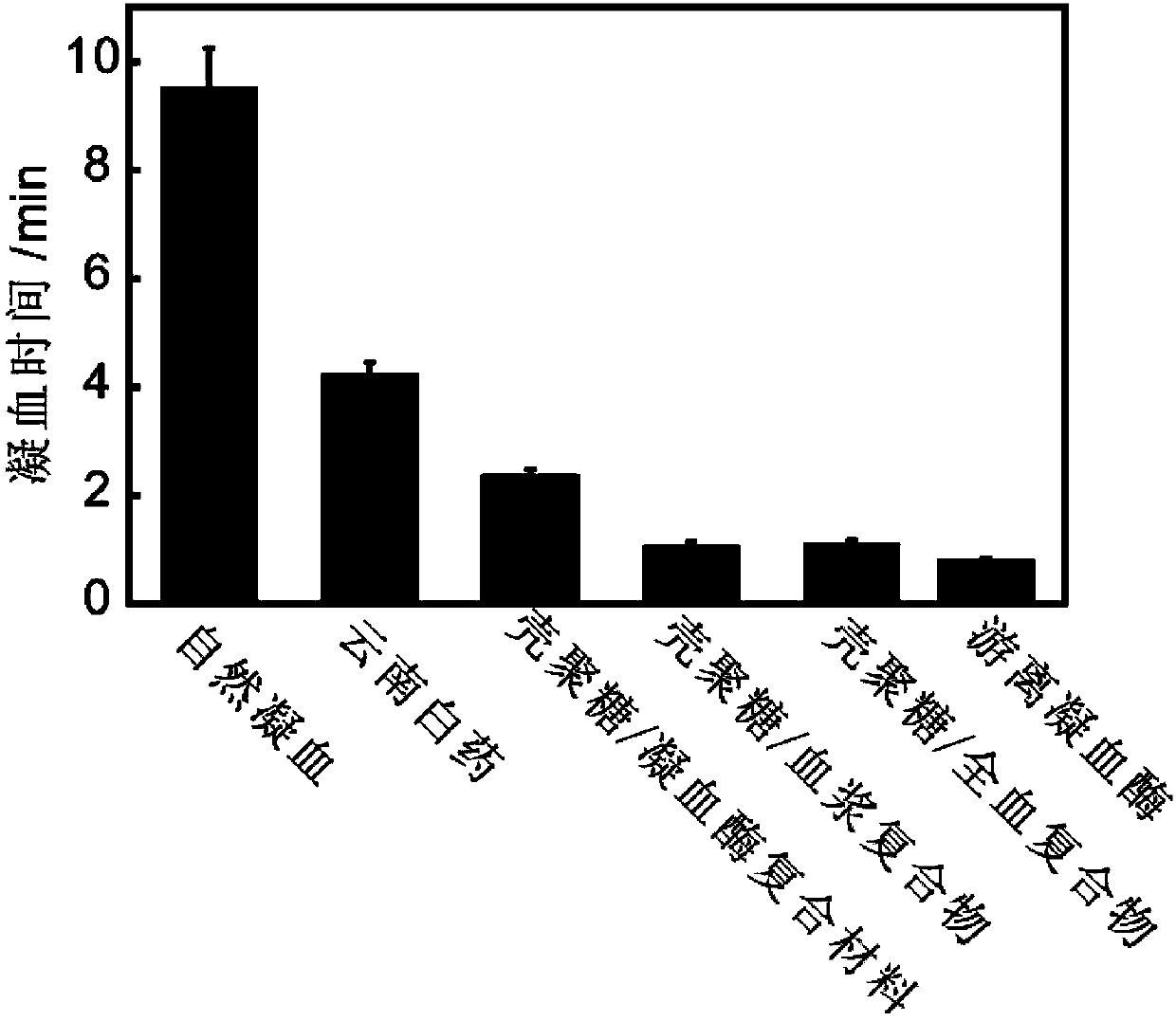
[0086] Preparation of chitosan / whole blood complex: mix 50mg chitosan (molecular weight 150,000-200,000) with 100ul whole blood (according to the mass ratio of 1:2), add calcium ions to make the concentration 5mM, 25℃ Mix for half an hour to obtain a chitosan / whole blood composite material, which is vacuum-dried at 30° C. for 5 hours to obtain a chitosan / whole blood composite.
[0087] Determination of the coagulation effect of the chitosan / whole blood complex: Grind the chitosan / whole blood complex, and conduct an in vitro coagulation experiment at 25°C, and place the whole blood in a water bath at 25°C for half an hour in advance. Take 50mg chitosan / whole blood complex, then add 40ul of 0.2M CaCl 2 , finally add 2ml of constant temperature whole blood to investigate its coagulation effect, and the time when the whole blood coagulates is recorded as the coagulation time of the chitosan / whole blood complex. Repeat the above-mentioned steps of measuring coagulation time three ...
Embodiment 3
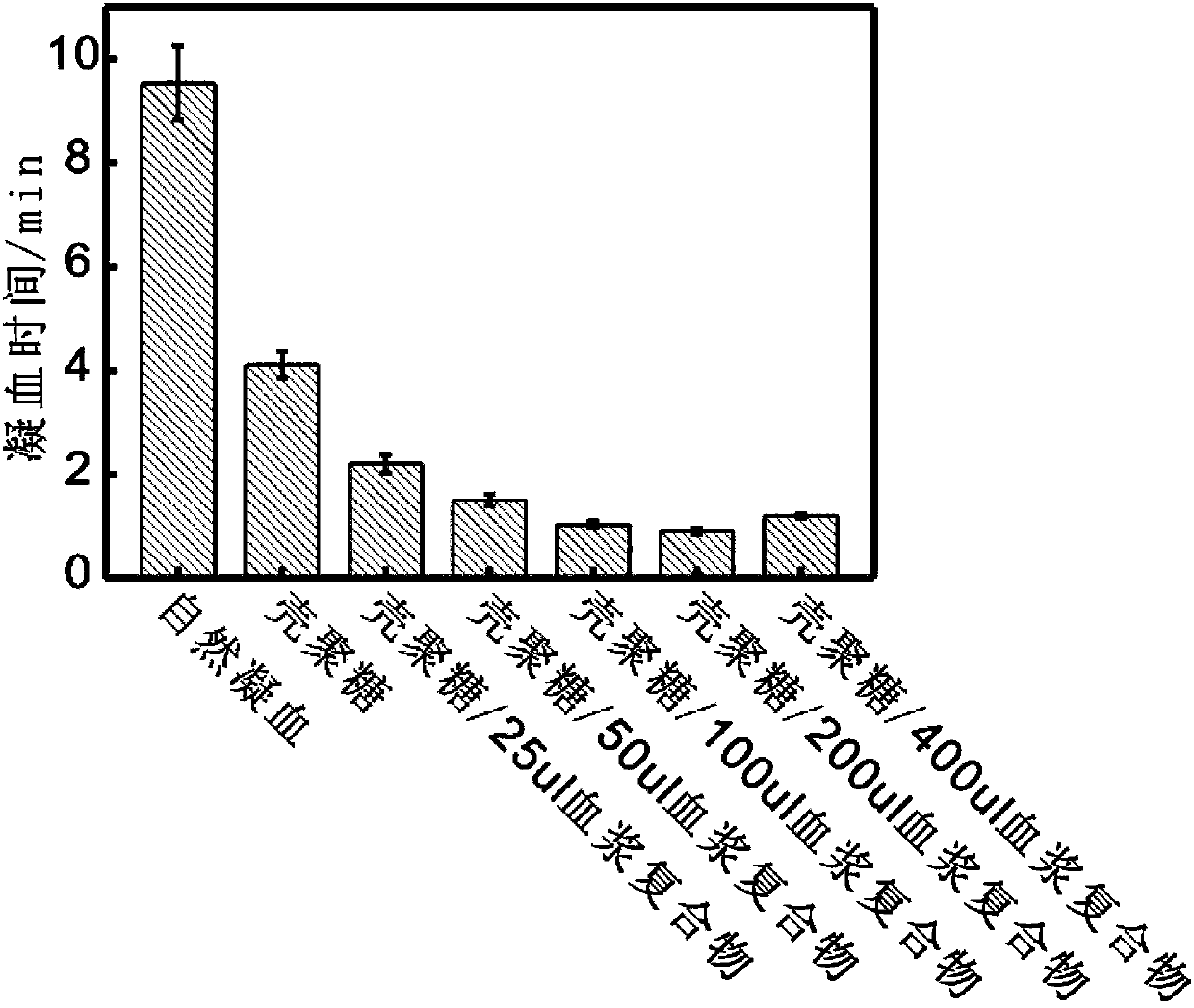
[0097] Preparation of different ratios of chitosan / plasma complexes: Mix 50mg chitosan (molecular weight 150,000-200,000) with 25ul, 50ul, 100ul, 200ul, 400ul anticoagulated plasma (1:0.5, 1:0, respectively) 1, 1:2, 1:4, 1:8 mass ratio), add calcium ions to make the concentration 5mM, and mix for half an hour at 25°C to prepare chitosan / 25ul plasma composite material and chitosan / 50ul plasma composite material, chitosan / 100ul plasma composite material, chitosan / 200ul plasma composite material, chitosan / 400ul plasma composite material were vacuum-dried at 30°C to obtain chitosan respectively / 25ul plasma complex, chitosan / 50ul plasma complex, chitosan / 100ul plasma complex, chitosan / 200ul plasma complex, chitosan / 400ul plasma complex.
[0098] Determination of coagulation effect of chitosan / plasma complexes with different ratios: In vitro coagulation experiment was carried out at 25°C, and the whole blood was kept in a water bath at 25°C for half an hour in advance. Take 50mg ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com