Recombinant nitrilase, coding gene, mutant, engineering bacteria and application thereof
A technology of genetically engineered bacteria and nitrilase, applied in the field of nitrilase, can solve problems such as high cost, environmental pollution, and harsh reaction conditions, and achieve good regioselectivity and catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Acidovorax facilis (Acidovorax facilis) ZJB09122 strain cells were preserved in liquid nitrogen, and genomic DNA was extracted with a genome extraction kit (FastDNA SPIN Kit soil). Using the genomic DNA as a template, the upstream primer 1AcN1(F)5 PCR amplification was carried out under the action of '-ATGGTTTCGTATAACAGCAAG-3' and downstream primer 2AcN1(R) 5'-CTACTTTGCTGGGACCGG-3'.
[0053] The amount of each component in the PCR reaction system (50 μL): 10 μL of 10*Pfu DNA Polymerase Buffer (Takara), 1 μL of 2.5 mM dNTP mixture (2.5 mM each of dATP, dCTP, dGTP and dTTP), cloning primer 1 and primer at a concentration of 25 μM 2 1 μL each, 1 μL of genomic DNA, 1 μL of 5 U / μL Pfu DNA Polymerase (Takara), 40 μL of nucleic acid-free water.
[0054] Using Biorad’s PCR instrument, the PCR reaction conditions are: pre-denaturation at 95°C for 5 minutes, then enter the temperature cycle at 94°C for 50 seconds, 55°C for 1.5 minutes, and 72°C for 2 minutes, a total of 35 cycles...
Embodiment 2
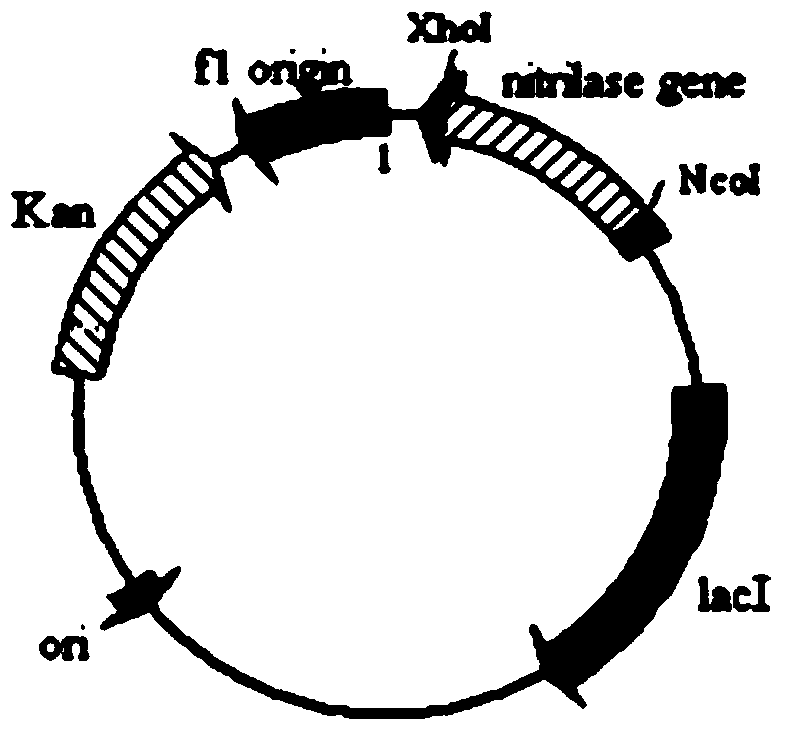
[0057] Design expression primer (upstream primer 3AcN1 (F) 5'-AAT according to embodiment 1 analysis result GGATCC ATGGTTTCGTATAACAGCAAG-3', downstream primer 4 AcN1(R)5'-AGG GTC GAC CTACTTTGCTGGGACCGG-3'), and Nco I and Xho I restriction enzyme sites were introduced into primer 3 and primer 4, respectively. Under the triggering of primer 3 and primer 4, high-fidelity Pyrrobest DNA polymerase was used to amplify to obtain a 1116bp nitrilase gene fragment (the nucleotide sequence is shown in SEQ ID NO: 2), and after sequencing, use The amplified fragment was double-digested with Nco I and Xho I restriction enzymes, and the fragment was ligated with the expression vector pET28b treated with the same restriction endonuclease using T4 ligase to construct the expression vector pET28b(+) -AcN1. Transform the constructed expression vector pET28b(+)-AcN1 into Escherichia coli BL21(DE3), spread it on a solid medium plate containing kanamycin (Kan) LB with a final concentration of 5...
Embodiment 3
[0061] The recombinant Escherichia coli BL21(DE3) / pET28b(+)-AcN1 verified in Example 2 containing the expression vector pET28b(+)-AcN1 was subjected to site-directed mutation F168V (the F at position 168 was mutated to V, and the conventional gene Engineering operations add a histidine tag to the C-terminus of the protein to obtain a mutant of the nitrilase), thereby obtaining a gene after site-directed mutation (the nucleotide sequence is shown in SEQ ID NO: 4, and the mutant gene encodes The nitrilase is abbreviated as AcN2, and its amino acid sequence is shown in SEQ ID NO: 3).
[0062] According to site-directed mutagenesis, using SEQ ID NO: 4 as a template, design upstream primer 5 F168V (F) 5'-GAGCACGTTCAGCCGCTGTCCAAAT-3' and downstream primer 6 F168V (R) 5'-CGGCTGAACGTGCTCCCAGCAGTTC-3', with SEQ ID NO: 4 Perform PCR amplification for the template (PCR reaction parameters: 94°C for 4min; 98°C for 10s, 55°C for 15s, 72°C for 6min, repeat 30 cycles; 72°C for 10min.). PCR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com