Preparation method of contrast medium intermediate triodoisophthalolyl chloride
The technology of isophthaloyl chloride and intermediate is applied in the field of preparation of contrast medium intermediate triiodoisophthaloyl chloride, can solve the problems of complicated operation process, serious industrial safety, problems and the like, and achieves the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
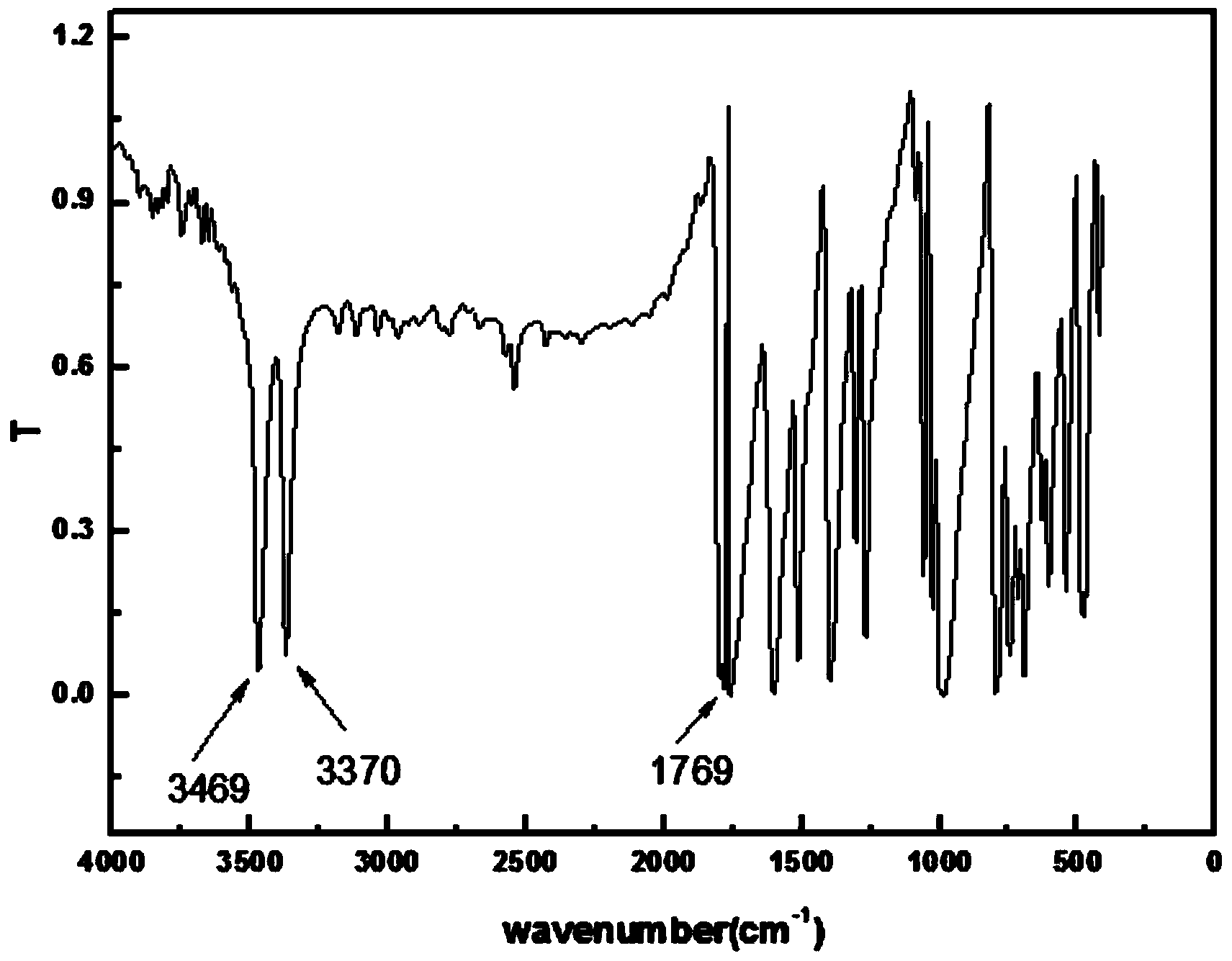
[0041] 10g (18mmol) of 5-amino-2,4,6-triiodoisophthalic acid, 3.3ml (45mmol) of thionyl chloride and 19.7mg (1.5%, 0.27mmol) of DMF were added to 600ml of isobutyl acetate , heated and reacted at 70°C for 6h, cooled to room temperature, poured the reaction liquid into 1000ml of ice water, slowly added saturated sodium bicarbonate solution until pH = 12, a large number of yellow solids were precipitated, filtered to obtain 12g of solids, and dissolved in 36ml of acetic acid iso Butyl ester was recrystallized and dried to obtain 10.5 g of 5-amino-2,4,6-triiodoisophthaloyl chloride with a yield of 99.1%.
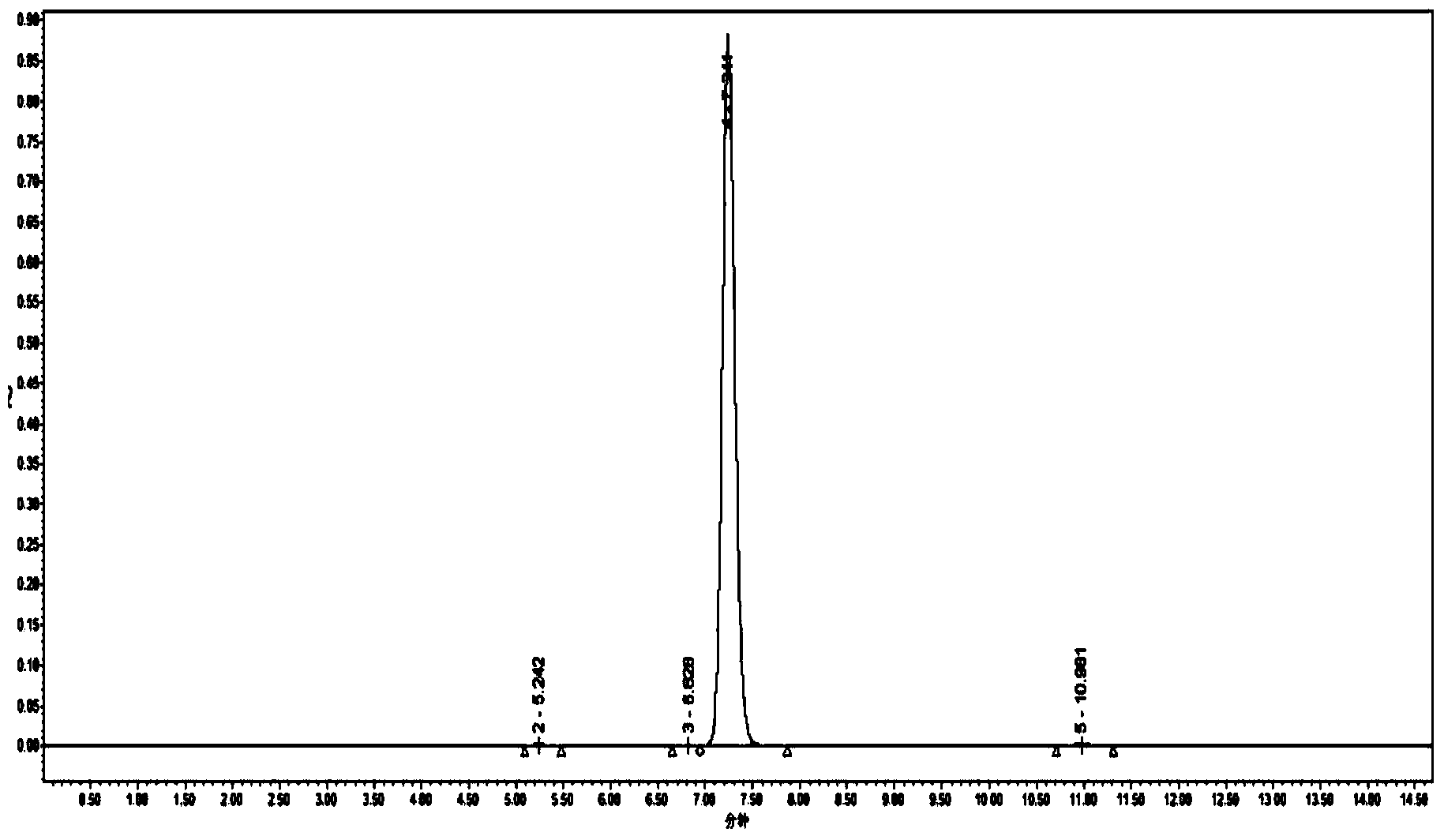
[0042] attached figure 1The high performance liquid chromatography (SYMMETRY C185μm, 4.6*250mm Column, column temperature 30°C, wavelength 254nm, water: acetonitrile = 20: 80). From the figure, it is the main product peak at about 7.24min, and there are three basically negligible impurity peaks at 5.24, 6.82, and 10.98min respectively. By the area normalization method, the p...
Embodiment 2
[0046] 30g (54mmol) of 5-amino-2,4,6-triiodoisophthalic acid, 9.4ml (135mmol) of thionyl chloride and 40mg of DMF (1%, 0.54mmol) were added to 1800ml of isobutyl acetate , heated and reacted at 70°C for 6h, cooled to room temperature, poured the reaction solution into 2000ml of ice water, slowly added saturated sodium bicarbonate solution until pH = 12, a large amount of yellow solid precipitated, filtered to obtain 37g of solid, and dissolved in 119ml of acetic acid iso The butyl ester was recrystallized and dried to obtain 31.6 g of 5-amino-2,4,6-triiodoisophthaloyl chloride with a yield of 98.8%.
Embodiment 3
[0048] 100 g (180 mmol) of 5-amino-2,4,6-triiodoisophthalic acid, 33 ml (450 mmol) of thionyl chloride and 260 mg of DMF (2%, 3.6 mmol) were added to 5143 ml of isobutyl acetate, Heat the reaction at 100°C for 4 hours, cool to room temperature, pour the reaction solution into 7000ml of ice water, slowly add saturated sodium bicarbonate solution until pH = 12, a large amount of yellow solid precipitates, filter to obtain 120g of solid, and weigh it with 360ml of isobutyl acetate Crystallized and dried to obtain 105.5 g of 5-amino-2,4,6-triiodoisophthaloyl chloride with a yield of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com