ph and oxidation-reduction dual-sensitive layer cross-linking nanoparticle as well as preparation method and application thereof
A nanoparticle, sensitive technology, applied in the fields of polymer chemistry and biomedical engineering, can solve the problems of fast clearance rate, poor targeting, poor controlled release effect, etc., to achieve improved enrichment and retention, low toxicity, and biocompatibility good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
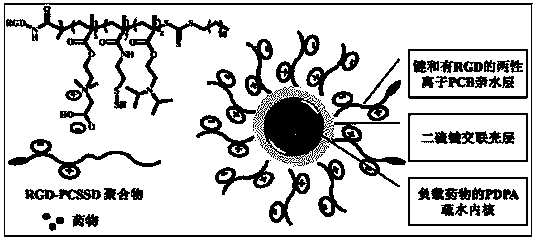
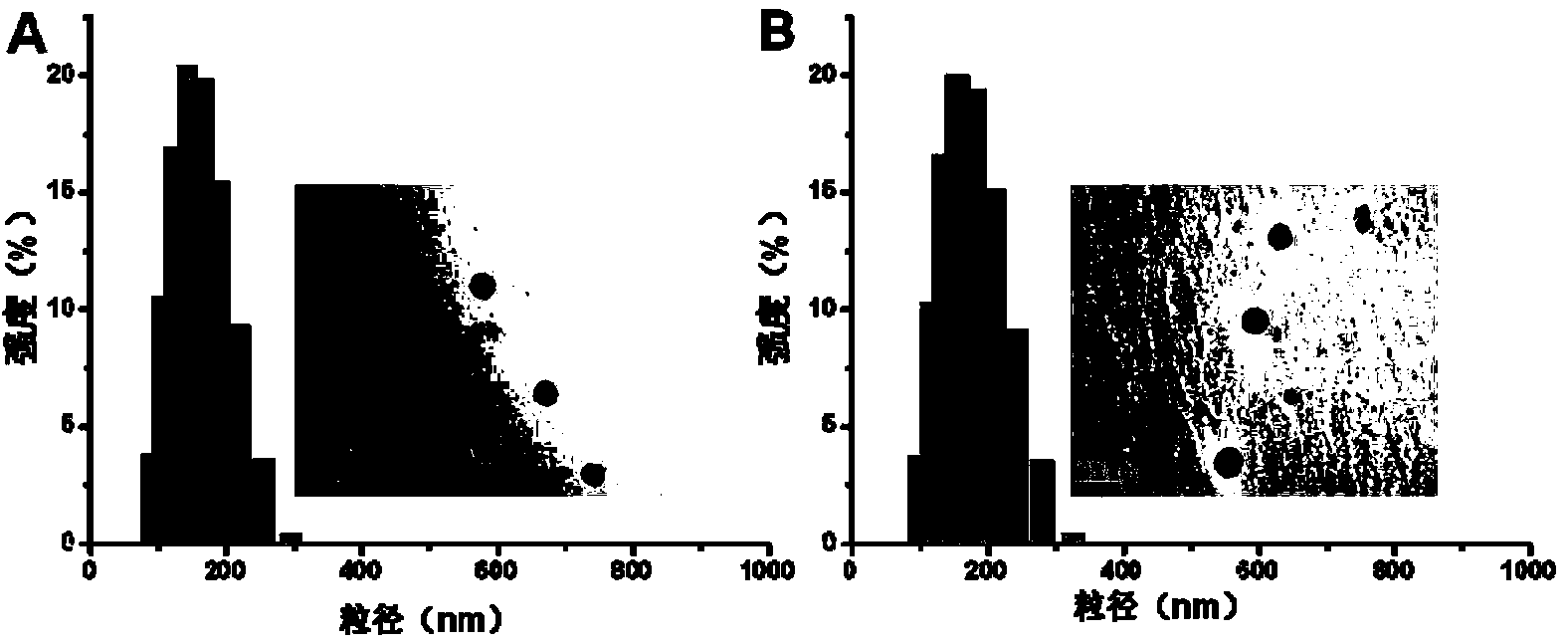
[0047] figure 1 It is a schematic diagram of double sensitive layer cross-linked nanoparticles of the embodiment of the present invention. from figure 1 It can be seen from the figure that the double sensitive layer cross-linked nanoparticles of the present invention have a three-layer structure, the outer layer is RGD with tumor targeting function and zwitterionic polymer PCB, the middle layer is a disulfide bond cross-linked shell layer, hydrophobic The drug is loaded in the inner core through the hydrophobic interaction with the hydrophobic segment PDPA. Example 1 (I-1)
[0048] The preparation method of triblock RGD-PCB-b-PDS-b-PDPA polymer comprises the steps:
[0049] (a) Preparation of PCB(tBU)-b-PDS-b-PDPA polymer
[0050] Add the reversible addition-fragmentation chain transfer polymerization (RAFT) chain transfer agent trithiododecyl-2-isopropionate (CTAm) (36.5 mg, 0.1 mM) and zwitterionic monomer in the shlenk reaction tube in sequence Carboxybetaine tert-buty...
Embodiment 2~ Embodiment 12
[0060] Device and operation are with embodiment 1, just by adjusting carboxylate betaine tert-butyl methyl methacrylate (CB-tBU), methacrylamide ethyl pyridine disulfide (DS) and methacrylic acid diisopropylamino The ratio of monomers such as ethyl ester (DPA) and chain transfer agent trithiododecyl-2-isopropionate (CTAm) obtains three-block polymers with different degrees of polymerization, as shown in Table 1:
[0061] Table 1: pH- and redox-sensitive polymers
[0062]
[0063] N a is the molar ratio of RGD to PCB-b-PDS-b-PDPA triblock polymer.
Embodiment 13
[0064] Embodiment 13 (I-13):
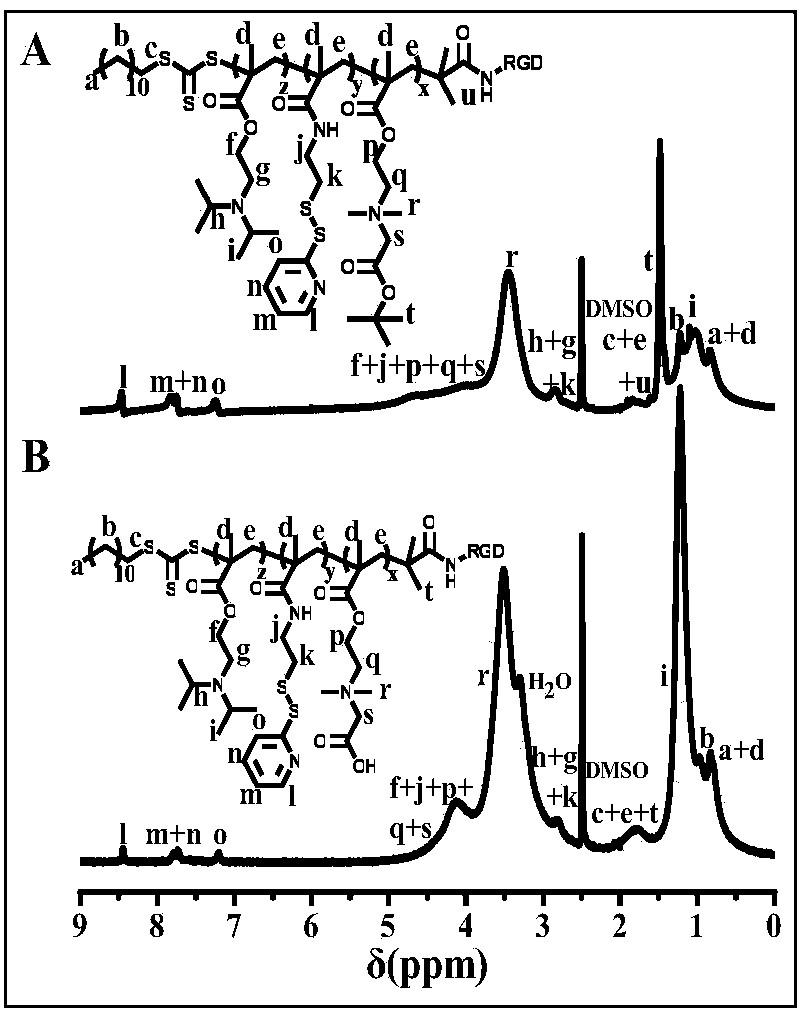
[0065] According to the method and device of Example 1 (a), replace CB-tBU with polyethylene glycol acrylate (RGD-PEG480-AC) bonded by RGD, and the number average molecular weight of the polyethylene glycol segment is 480 to obtain RGD-PEG480-AC. b-PDS-b-PDPA. NMR characterization of its composition RGD-PEG480 10 -b-PDS 10 -b-PDPA 25 (I-13), the subscript is the degree of polymerization of the corresponding monomer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com