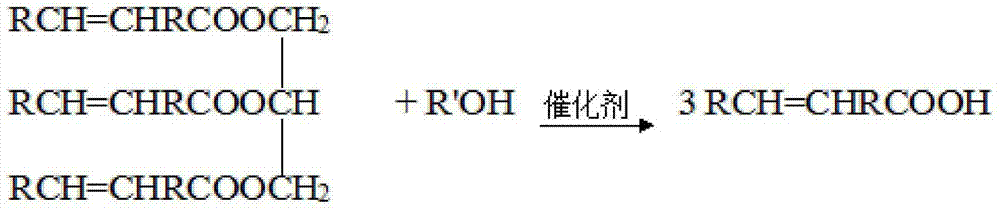Inflaming retarding bio-based polyhydric alcohol and preparation method thereof
A bio-based polyol, flame-retardant technology, applied in the direction of cyanide reaction preparation, organic compound preparation, carboxylate preparation, etc., can solve non-degradable problems, and achieve non-degradable, reduced dependence, enhanced phase Capacitance and Adhesion Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Step a: In a 250mL three-necked flask, add 6.7gKOH, 60mL methanol and 30mL water, stir and heat to 70°C, quickly add 29.9g tung oil, the reaction time is 2h, and after refining, 28.6g of light yellow liquid tungoleic acid, acid The value is 195.8mg / g;
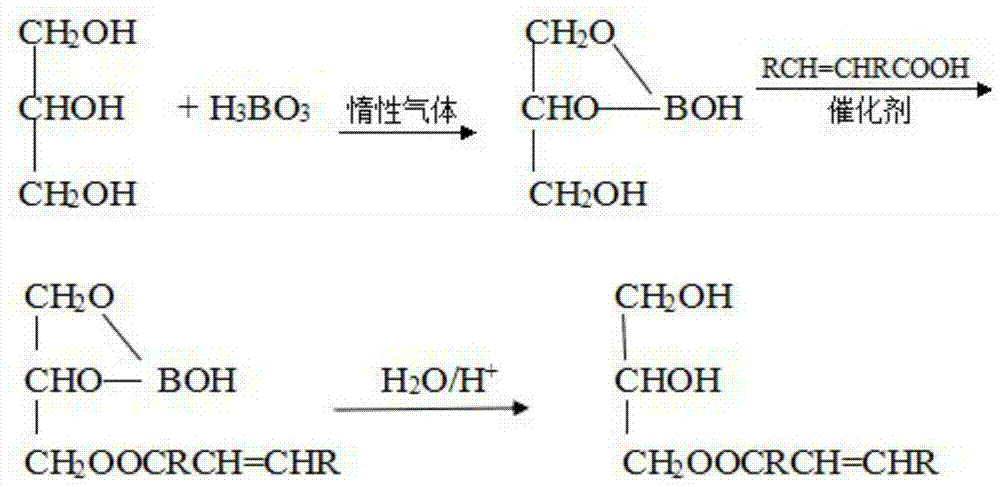
[0052] Steps b and c: Add 13.8g glycerol and 6.2g boric acid into a four-necked flask, under the protection of inert gas, control the temperature at 200°C, and the reaction time is 1.5h, then refine to obtain boric acid diglyceride, then add 28.7g tung oil acid and 0.3g p-toluenesulfonic acid, the temperature is controlled at 220°C, and after 2 hours of reaction, the glyceryl oleic acid borate is obtained. Glyceride product 35.2g;
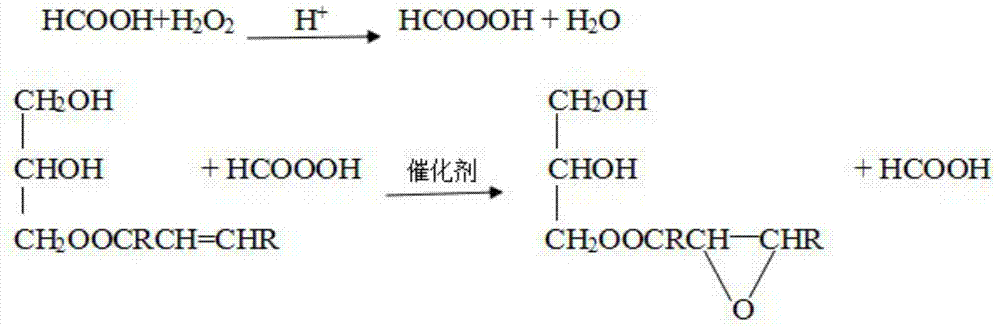
[0053] Step d: Take 20 g of monoglyceride oleic acid, add 1.48 g of formic acid (85%), and 0.1 mL of phosphoric acid into a 250 mL three-necked flask, stir, add 10.2 g of hydrogen peroxide (30%) dropwise, and react at 60 ° C for 6 h. After being refined, epoxy oleic acid ester was obtained,...
Embodiment 2
[0058] Step a: In a 250mL three-necked flask, add 7.8g NaOH, 70mL ethanol and 35mL water, stir and heat to 60°C, quickly add 34.9g tung oil, the reaction time is 1.5h, and after refining, 32.8g of light yellow liquid tungoleic acid is obtained, The acid value is 199.5mg / g;
[0059] Steps b and c: Add 16.5g glycerin and 9.3g boric acid into a four-neck flask, under the protection of inert gas, control the temperature at 180°C, and the reaction time is 2h, then refine to obtain boric acid diglyceride, then add 34.8g oleic acid and 0.4g of phosphoric acid, the temperature is controlled at 230°C, and after 1.5h of reaction to obtain boric acid glycerides, at room temperature, use 2mol / L hydrochloric acid solution to hydrolyze glycerol borates, and then obtain monoglycerides of oleic acid after refining 39.4g;
[0060] Step d: Take 25g of monoglyceride oleic acid, add 1.85g of formic acid (85%), and 0.1ml of phosphoric acid into a 250ml three-necked flask, stir, add 12.7g of hydro...
Embodiment 3
[0065] Step a: In a 250mL three-necked flask, add 7.8g NaOH, 70mL ethanol and 35mL water, stir and heat to 60°C, quickly add 34.9g tung oil, the reaction time is 1.5h, and after refining, 32.8g of light yellow liquid tungoleic acid is obtained, The acid value is 199.5mg / g;
[0066] Steps b and c: Add 16.5g glycerin and 9.3g boric acid into a four-neck flask, under the protection of inert gas, control the temperature at 180°C, and the reaction time is 2h, then refine to obtain boric acid diglyceride, then add 34.8g oleic acid and 0.4g of phosphoric acid, the temperature is controlled at 230°C, and after 1.5h of reaction to obtain boric acid glycerides, at room temperature, use 2mol / L hydrochloric acid solution to hydrolyze glycerol borates, and then obtain monoglycerides of oleic acid after refining 39.4g;
[0067] Step d: Take 25g of monoglyceride oleic acid, add 1.85g of formic acid (85%), and 0.1ml of phosphoric acid into a 250ml three-necked flask, stir, add 12.7g of hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com