Method for preparing 5-hydroxymethylfurfural through catalyzing fructose conversion by solid catalyst
A technology of hydroxymethyl furfural and solid catalyst, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the influence of catalytic activity, loss of active components, The problem of low thermal stability, etc., can achieve the effect of optimizing catalytic performance, improving selectivity, and improving effective diffusion and departure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
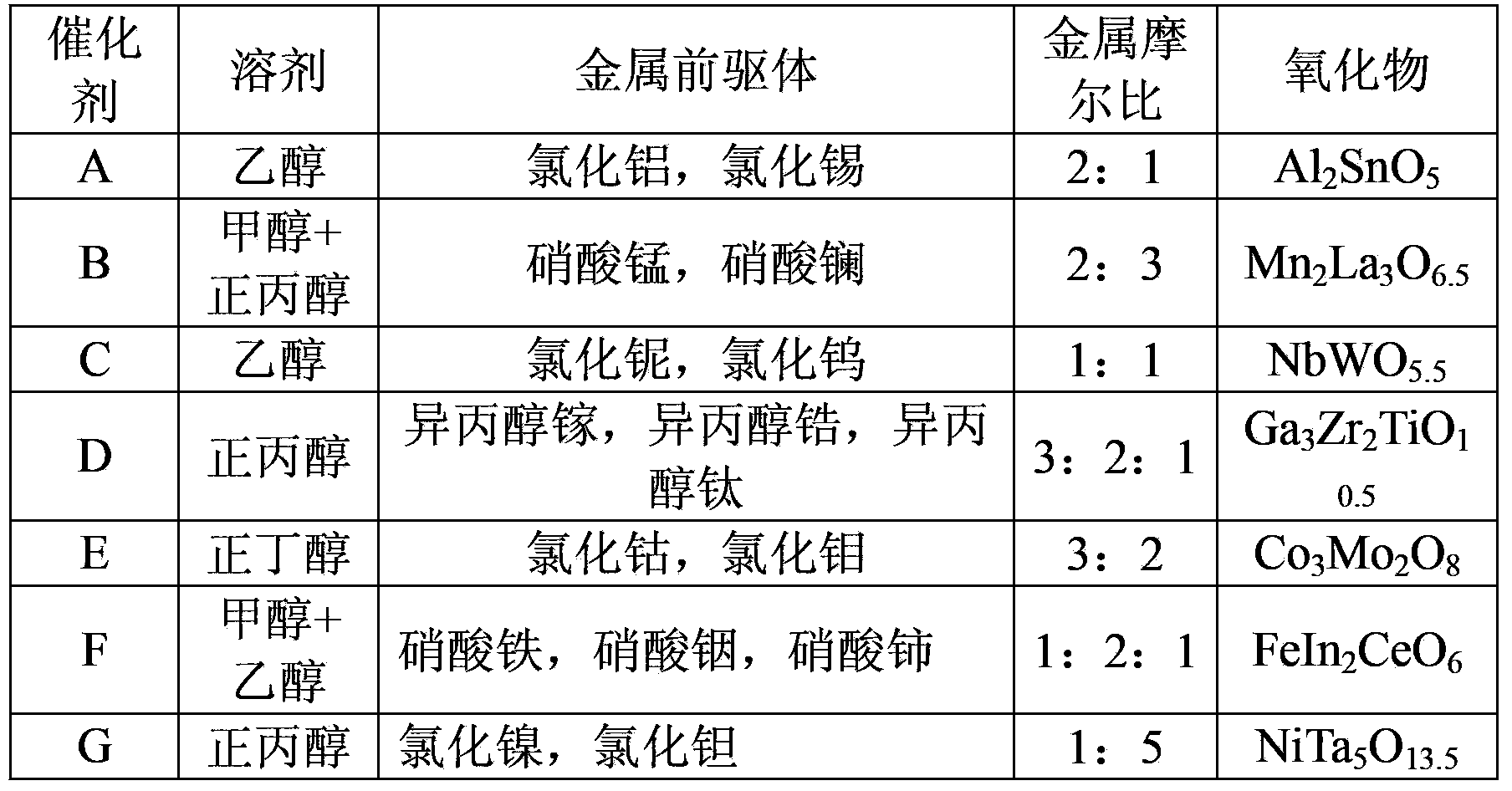
[0018] Embodiment one: the preparation of catalyst A
[0019] Get 5 grams of polyethylene glycol-polypropylene glycol-polyethylene glycol triblock polymer (EO 20 PO 30 EO 20 , Mav=3400) was dissolved in 50 g of methanol to prepare solution 1. 2.6 g of aluminum chloride and 2.6 g of tin tetrachloride were respectively added to solution 1, after vigorous stirring for 1 hour, 0.5 g of water was added, and stirring was continued for 1 hour to form a sol solution. Then the solution was left to stand for 7 days at 40 degrees to gradually form a gel. After the aged gel sample was baked at 450°C for 10 hours to remove the surfactant, the catalyst A:Al 2 SnO 5 .
Embodiment 2
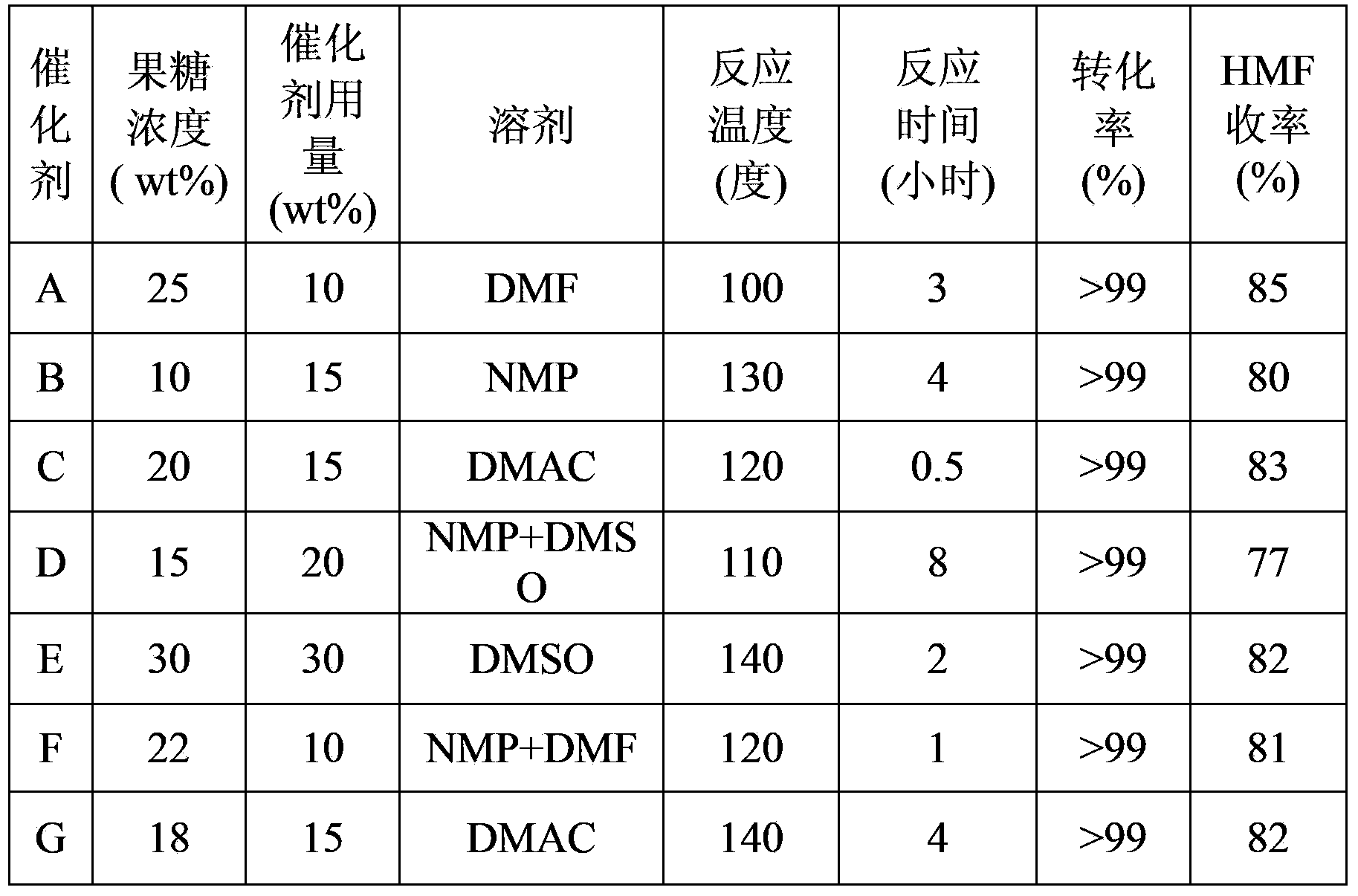
[0020] Embodiment 2: Dehydration of fructose to prepare HMF reaction activity determination
[0021] Get 5 grams of fructose, 0.5 grams of catalyst A and 20 grams of DMF into a 100 milliliter flask, stir magnetically, heat to 100 degrees, and stop the reaction after 8 hours of reaction. The catalyst was separated by centrifugation, and the upper reaction mixture was taken for analysis, wherein the raw material was quantitatively analyzed by liquid chromatography, and the product was quantitatively analyzed by gas chromatography. The reaction results are shown in Table 2.
Embodiment 3
[0022] Embodiment three: preparation and catalytic activity of catalyst B-G
[0023] Except for the types and proportions of the metal precursors used, the preparation method of the other catalysts is the same as in Example 1, and the compositions of catalysts B-G are shown in Table 1. The method for measuring the activity of the catalyst is the same as in Example 2. The results are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com