Preparation method of 3,5-dichloro-4-methylbenzoic acid
A technology of toluic acid and p-toluic acid, applied in 3 fields, can solve the problems of not being able to be used in large-scale production, high raw material prices, high operation requirements, etc., to reduce the difficulty of separation and purification, easy to obtain raw materials, and simple production process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: 3, the preparation method of 5-dichloro-4-methylbenzoic acid
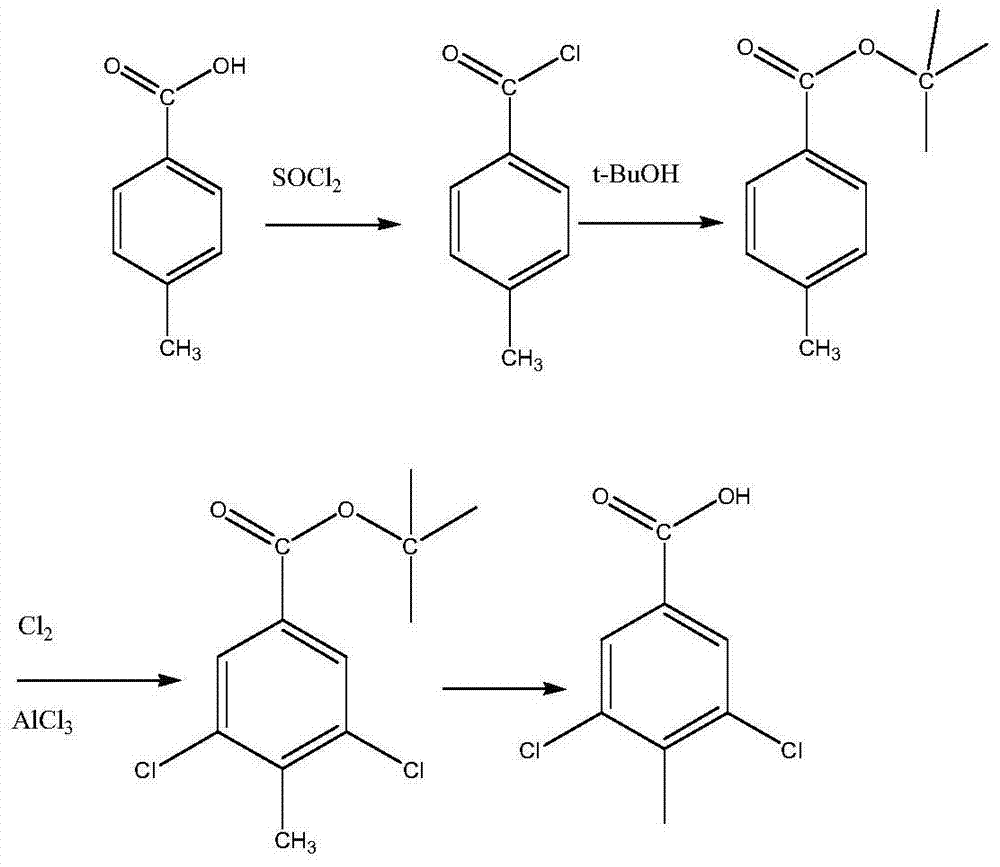
[0028] The invention uses p-toluic acid as a raw material, undergoes esterification, chlorination and hydrolysis in sequence to obtain 3,5-dichloro-4-methylbenzoic acid product. The reaction equation is:
[0029]
[0030] The preparation method of 3,5-dichloro-4-methylbenzoic acid disclosed by the present invention comprises the following steps (the volume or volume involved in it all adopts mL as the unit; the mass or weight involved in it all adopts g as unit):
[0031] A. Install a mechanical stirrer on a three-necked flask with 1000 parts by volume, add 700 parts by weight of thionyl chloride and 200 parts by weight of p-toluic acid, add 3 drops of DMF dropwise, heat up and reflux for 5 hours, and there will be no more bubbles. stop, 68 degrees of reflux temperature; steam excess thionyl chloride under normal pressure, steam to 100 degrees of liquid temperature and stop, cool to 30 deg...
Embodiment 2
[0034] Embodiment 2: The ratio selection research of p-toluic acid (A) and thionyl chloride (B), relevant data results are summarized in the following table:
[0035] A / B (molar ratio)
[0036] It can be seen from the above table that if too little thionyl chloride is used, a large amount of raw material p-toluic acid will remain, and in the next esterification process, the raw material will still remain in it, and the purity of the final product will be greatly reduced. After purification If the loss is too much, the usage of thionyl chloride should not be too much, too much will cause waste. Therefore, the molar ratio of p-toluic acid (A) to thionyl chloride (B) was selected as 1:4, which was used as the optimal condition.
Embodiment 3
[0037] Embodiment 3: The ratio selection research of p-toluic acid (A) and tert-butanol (C), the relevant data are summarized in the following table:
[0038] A / C (molar ratio)
[0039] Since the reaction speed of acid chloride itself and alcohols is relatively fast, a large excess of tert-butanol is not required to increase the reaction speed. It can be seen from the above table that in order to ensure the complete esterification of the acid chloride, the optimal molar ratio of p-toluic acid (A) to tert-butanol (C) is selected as 1:1.1, and the reaction results also prove that this ratio is sufficient to completely esterify the acid chloride. transform.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com