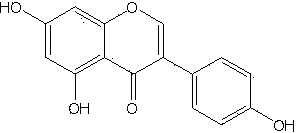
Drug resistant Staphylococcus aureus combined drug and application
A staphylococcus, golden yellow technology, applied in the field of pharmacy, can solve the problems of reducing the concentration of antibiotics, limiting the application of norfloxacin, unable to play an antibacterial effect, etc., and achieving the effect of reducing the antibacterial concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Isolation of synergistic compound soybean isoflavones (genistein) from plants
[0037] Sophora japonica (( Sophora moorcroftiana) aboveground part 1.0 kg, dried and pulverized with 95% ethanol 45 o Extracted three times at C, combined the extracts, concentrated under reduced pressure to obtain 124 g of extract, extracted with petroleum ether and water in turn, and the remaining part weighed 35 g after the solvent was evaporated. The 35 g extract was mixed with 200-300 mesh silica gel, and gradient eluted with petroleum ether and ethyl acetate (the initial ratio was 8:2, and finally washed with pure ethyl acetate), and each 60 mL was used as one split. The 21st-34th fractions were combined and recorded as fractions Fr 03-21 after recovering the solvent, with a total of 1.7 g. Fraction 21 was mixed, first washed with chloroform for two column volumes, and then eluted with chloroform-acetone (9:1), each 20 mL was a fraction. Fractions 32-40 were collected...
Embodiment 2
[0038] Example 2. Isolation of soy isoflavones from soybean products commercial food raw material Bioisogen?
[0039] Food raw material Bioisogen? was purchased from Jichun Factory, Amorepacific Co. Ltd., Korea. 100 g of Bioisogen® raw material was extracted three times with 1 liter of acetone or chloroform-methanol (1:1) solvent, and the solvent was evaporated to dryness to obtain about 23 g of light yellow amorphous solid. Get 1 gram of it and use silica gel column chromatography, elute with chloroform-methanol (95:5) mobile phase, every 8 mL is a flow fraction, combine the 8th-25th flow fraction, evaporate to dryness to obtain 300 mg soybean isoflavones (genistein ).
Embodiment 3
[0040] Example 3. Determining the optimal ratio of compound soybean isoflavone (genistein) and norfloxacin in combination
[0041] Soy isoflavones and norfloxacin were respectively prepared mother liquors, and the mother liquors were pipetted and mixed according to the ratios shown in the table below to obtain 12 compositions numbered 1-12, each with a volume of 120 μL. Pipette 100 μL of the composition numbered 1 and add it to 1.5 mL broth to obtain the stock solution of composition 1. Treat compositions 2-11 in the same way. Composition 12 does not contain genistein and norfloxacin, and it is negative control. Each composition was subjected to an antibacterial test and the MIC value of each composition was recorded (as shown in Table 2).
[0042] Table 2. Compositions of Compositions 1-12 and their MIC values against drug-resistant Staphylococcus aureus
[0043] Composition number Ratio of G to N Combination drug concentration (μg / mL) The MIC value (μg / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com