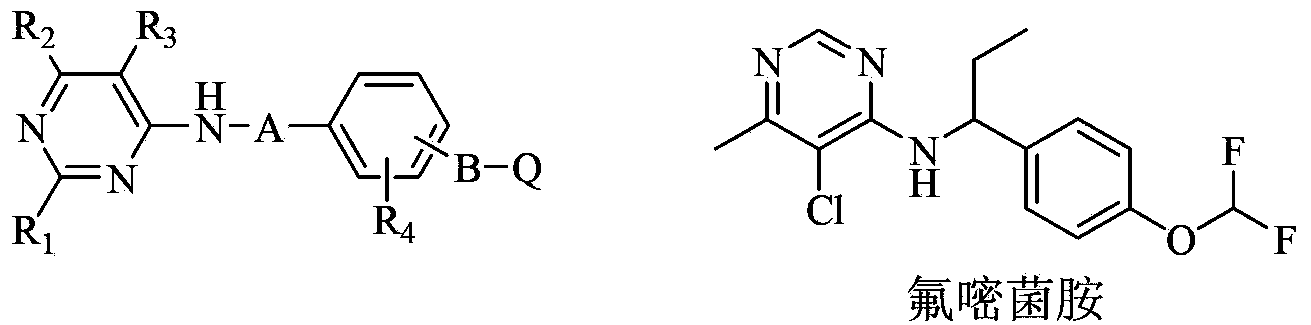
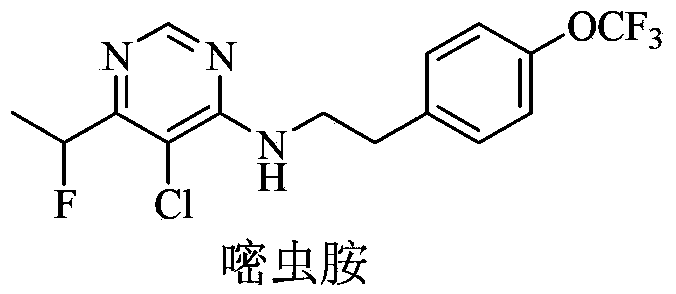
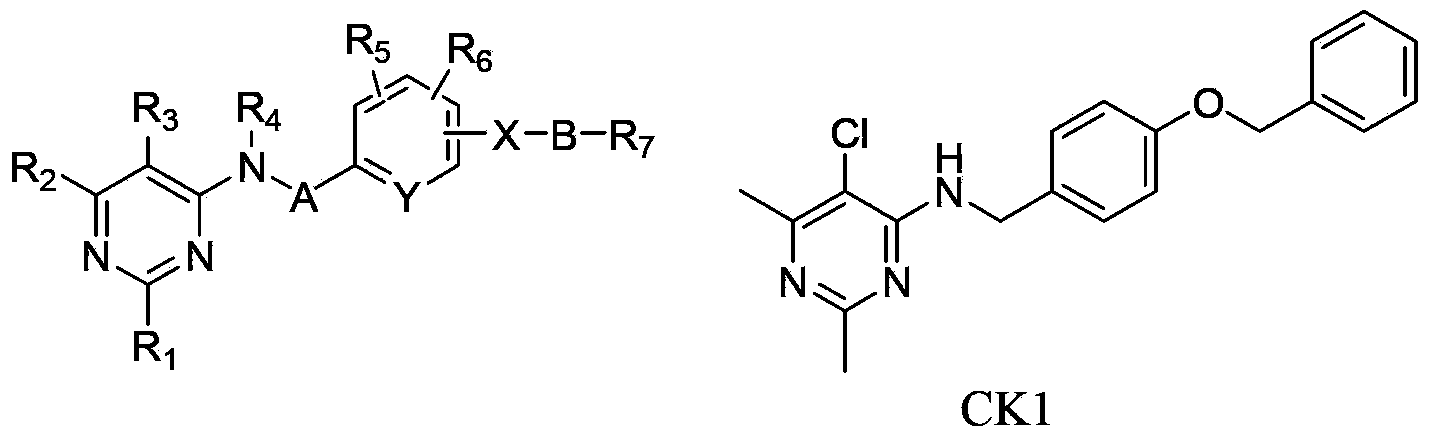
Substituted pyrimidinamine compound and use thereof
A technology of pyrimidine amines and compounds, applied in the fields of chemicals, applications, and organic chemistry for biological control, which can solve the problems that the structure of pyrimidine ethylamine or propylamine compounds has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0453] Example 1: Preparation of intermediate 4,5-dichloro-6-methylpyrimidine (II-1)
[0454] 1) Preparation of ethyl 2-chloro-acetoacetate
[0455]
[0456] Slowly add 34.26g (0.25mol) sulfonyl chloride solution in dichloromethane to 30.00g (0.23mol) of ethyl acetoacetate in 80ml of dichloromethane under stirring at room temperature. After about 1.5 hours, a large amount of gas will be produced. , continue to stir the reaction at room temperature for 5-7 hours, after the reaction is monitored by TLC, dichloromethane solvent and excess sulfuryl chloride are evaporated under reduced pressure to obtain 30 g of light yellow liquid.
[0457] 2) Preparation of 4-hydroxy-5-chloro-6-methylpyrimidine
[0458]
[0459] Slowly add 8.80g (0.16mol) sodium methoxide methanol solution dropwise to 11.30g (0.11mol) formamidine acetate solution in 50ml methanol under stirring at room temperature, and stir at room temperature for 2h after dropping. Then add 11.17g (0.068mol) ethyl 2-chl...
Embodiment 2
[0463] Embodiment 2: the preparation of intermediate 2-(4-(4-methylbenzyloxy)phenyl)ethylamine
[0464] 1) Preparation of 2-(4-(4-methylbenzyloxy)phenyl)acetonitrile
[0465]
[0466] Add 14.05g (0.1mol) of 4-methylbenzyl chloride and 15.96g (0.12mol) of p-hydroxyphenylacetonitrile into 200ml of butanone, add 27.60g (0.2mol) of potassium carbonate, heat to reflux under stirring, and react for 4-10 hours , after the reaction was monitored by TLC, the solvent was evaporated under reduced pressure, extracted with 300ml of ethyl acetate, washed with 5% aqueous sodium hydroxide solution and 50ml of saturated saline successively, and the residue after precipitation was separated by column chromatography to obtain a white solid 18.70 g, yield 79.0%, melting point 85-87°C.
[0467] 2) Preparation of 2-(4-(4-methylbenzyloxy)phenyl)ethylamine
[0468]
[0469] A mixture of 2.37g (0.01mol) of the intermediate 2-(4-(4-methylbenzyloxy)phenyl)acetonitrile, Raney nickel (1.0g), 25% a...
Embodiment 3
[0470] Embodiment 3: Preparation of 2-(4-(2-methylbenzyloxy)phenyl)ethylamine hydrochloride
[0471] 1) Preparation of N-Boc-4-hydroxyphenethylamine
[0472]
[0473] Dissolve 11.3g (0.1mol) of 4-hydroxyphenethylamine in 80ml of tetrahydrofuran, add 10.08g (0.12mol) of sodium bicarbonate and 50ml of water in sequence, and add 21.80g (0.1mol) of di-tert-butyl dicarbonate dropwise under stirring at room temperature Esters, dropwise, continue to react for 4-10 hours, after TLC monitors the reaction, evaporate the solvent under reduced pressure, extract (3×50ml) ethyl acetate, wash with saturated brine 50ml, and the residue after precipitation is separated by column chromatography 17.15 g of white solid was obtained, the yield was 80.5%, and the melting point was 67-68°C.
[0474] 2) N-Boc-2-(4-(2-methylbenzyloxy)phenyl)ethylamine
[0475]
[0476] Add 2.37g (0.01mol) N-Boc-4-hydroxyphenethylamine and 1.41g (0.01mol) 2-methylbenzyl chloride into 50ml butanone, add 2.76g (0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com