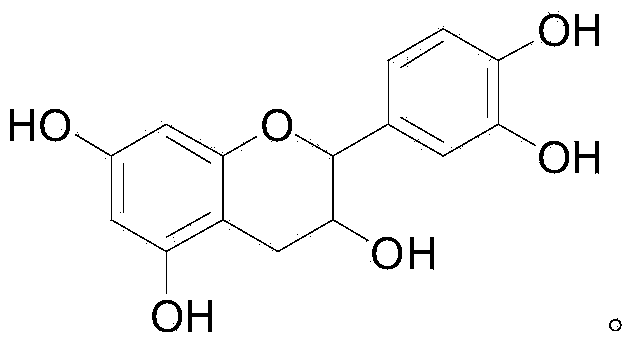
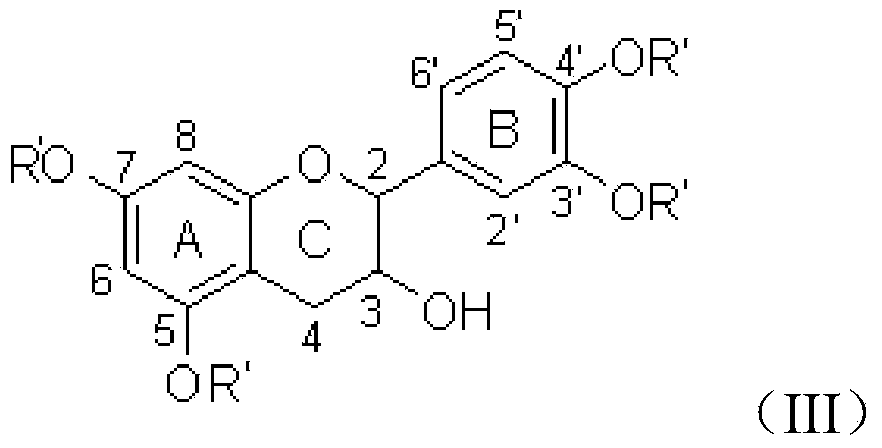
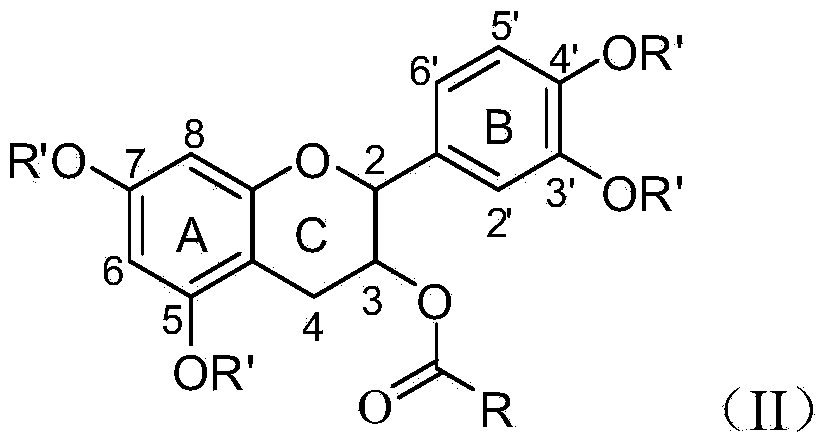
Method for preparing 3-ester group catechin
A technology of ester catechin and catechin is applied in the field of preparation of 3-ester catechin, can solve the problems of high cost, unsuitability for industrialized production, etc., achieves good selectivity, avoids toxicity and corrosive reagents and reactions mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Catechin (29 mg), propionic anhydride (59 mg) and triethylamine (45 mg) were stirred and reacted at room temperature for 20 hours, extracted with ethyl acetate after the reaction, and chromatographed (eluent ethyl acetate mixed with petroleum ether) solvent) to obtain 3',4',5,7-tetrapropyl catechin (yield: 78%) after purification. Then react with lauryl chloride (44mg) and pyridine (24mg) for 24 hours, then react with hydrazine hydrate (40mg) for 8 hours, extract with ethyl acetate after the reaction, and obtain 3-dodecyl Ester catechins 72%.
[0038] Structure determination data: 1 H NMR(400MHz,DMSO)δ9.35(s,1H),9.08(s,1H),8.96(s,1H),8.90(s,1H),6.71(d,J=1.6Hz,1H),6.68 (d,J=8.4Hz,1H),6.57(dd,J=8.4,2.0Hz,1H),5.93(d,J=2.0Hz,1H),5.77(d,J=2.0Hz,1H),5.11 (q, J=6.0Hz, 1H), 4.90(d, J=6.4Hz, 1H), 2.64(dd, J=16.4, 5.2Hz, 1H), 2.54-2.46(m, 1H), 2.17(td, J=7.2,3.2Hz,2H), 1.42-1.33(m,2H), 1.32-1.03(m,16H), 0.86(t,J=7.2,3H). The mass spectrum shows the dehydrogenation molecular i...
Embodiment 2
[0040]Catechin (29 mg), acetic anhydride (46 mg) and tripropylamine (57 mg) were stirred and reacted at room temperature for 20 hours, extracted with ethyl acetate after the reaction, and purified by chromatography to obtain 3',4',5,7 - Tetraethyl catechin (yield 75%). Then react with octanoyl chloride (24mg) and pyridine (32mg) for 24 hours, then react with hydrazine hydrate (40mg) for 8 hours, extract with ethyl acetate after the reaction, and obtain 3-octyl catechin after chromatographic purification 75%. The structure detection data is consistent with the prior art (mass spectrum shows that the dehydrogenation molecular ion peak of the main component is 414.88).
[0041] The structure determination data of 3',4',5,7-tetraethyl catechin: 1 H NMR (400MHz, DMSO-d6) δ7.37-7.25 (m, 3H), 6.61 (d, J = 2.8Hz, 1H), 6.60 (d, J = 2.4Hz, 1H), 4.87 (d, J = 8.0Hz, 1H), 4.05-3.95(m, 1H), 2.78(dd, J=16.4, 5.6Hz, 1H), 2.57-2.48(m, 1H), 2.30(s, 3H), 2.29(s, 6H ), 2.24(s,3H). The mass sp...
Embodiment 3
[0043] Catechin (29 mg), butyric anhydride (71 mg) and N,N-diisopropylethylamine (52 mg) were stirred and reacted at room temperature for 20 hours. After the reaction was completed, they were extracted with ethyl acetate and purified by chromatography to obtain 3 ',4',5,7-tetrabutyl catechin (74% yield). Then react with octanoyl chloride (24mg) and pyridine (32mg) for 24 hours, then react with hydrazine hydrate (40mg) for 8 hours, extract with ethyl acetate after the reaction, and obtain 3-octyl catechin after chromatographic purification 70%. The structure detection data is the same as in Example 2.
[0044] 3',4',5,7-tetrabutyl catechin structure determination data: 1 H NMR (400MHz, DMSO) δ7.39-7.24 (m, 3H), 6.60 (d, J = 2.0Hz, 1H), 6.58 (d, J = 2.0Hz, 1H), 4.88 (d, J = 8.0Hz ,1H),4.05-3.95(m,1H),2.77(dd,J=16.4,5.6Hz,1H),2.63-2.46(m,9H),1.73-1.59(m,8H),1.02-0.92(m , 12H). The mass spectrometry showed that the molecular ion peak of sodium addition was 593.17.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com