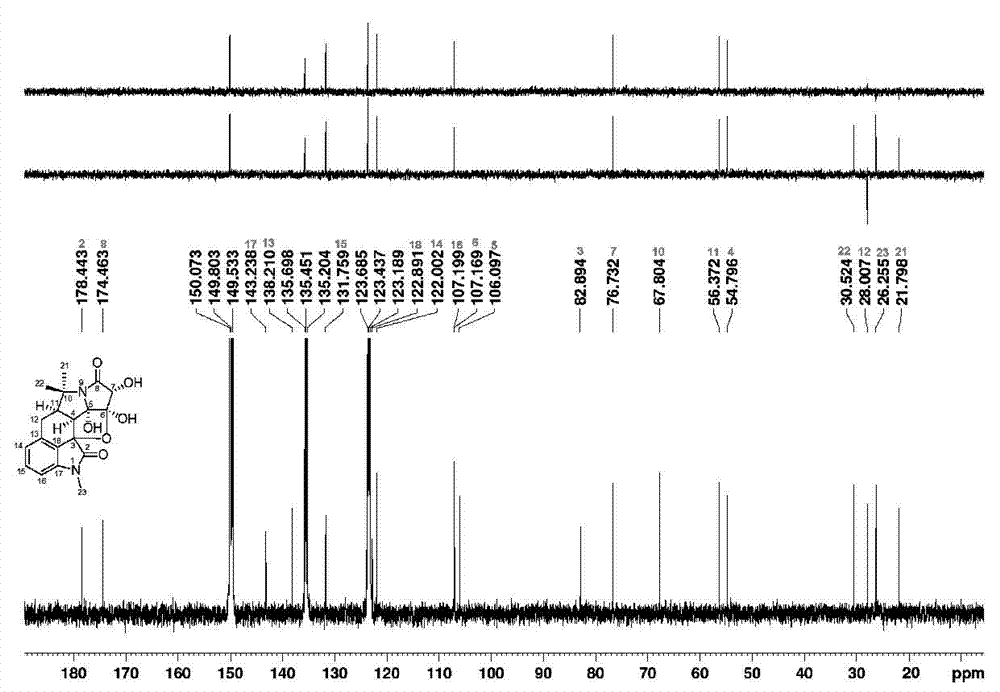
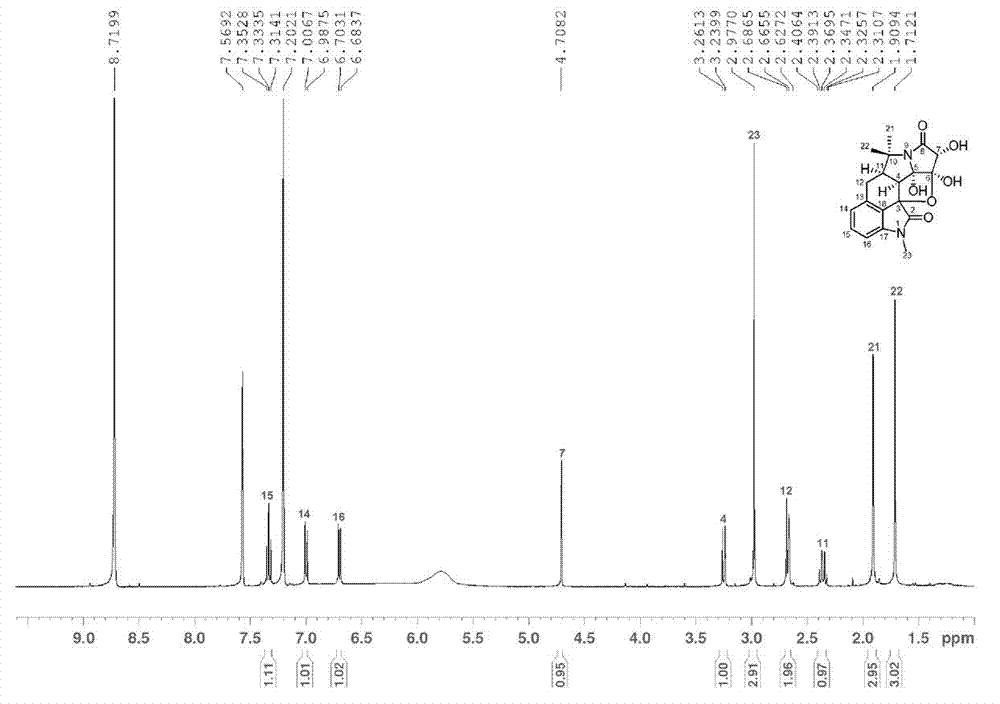
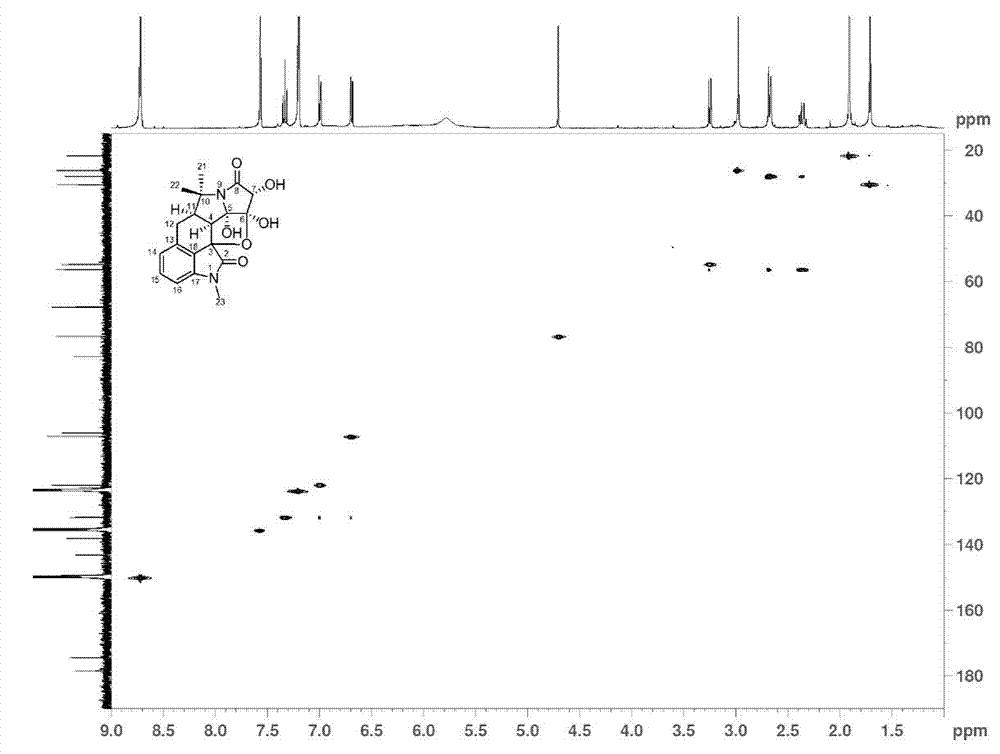
Hexacyclic alkaloid compound, preparation method and application thereof
A technology of alkaloids and compounds, applied in the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of the hexacyclic alkaloid compound of the present invention comprises the following steps:
[0032] A. Solid fermentation: Cultivate the strain in potato dextrose agar medium at 28°C for 7 days, inoculate it into 100ml potato dextrose liquid medium (cut 200 g potatoes into small pieces, add water and boil them until rotten (boil for 20-30 minutes, Potato cubes can be pierced by a glass rod), filter with eight layers of gauze, heat, add 5 g of agar, continue to heat and mix well, after the agar is dissolved, add 20 g of glucose, stir evenly, and cool down slightly Make up water to 1000ml, divide into test tubes or Erlenmeyer flasks, stopper and bandage, (121°C) for about 20 minutes after sterilization, take out the test tubes and place them on an inclined plane or shake well, cool down and store them in 250ml Erlenmeyer flasks, at Cultivate for 5 days at 28°C under the condition of rotation and shaking at 180rpm to obtain strains for fermentation,...
Embodiment 1
[0057] ------Solid fermentation and extract extraction:
[0058] The strain was cultured in potato dextrose agar medium for 7 days at a culture temperature of 28 °C; the agar plug was inoculated into a 250 mL Erlenmeyer flask containing 100 mL of potato dextrose, and cultured at 28 °C under the condition of rotating and shaking at 180 rpm After 5 days, the strains for fermentation were obtained. Solid fermentation was carried out in 500 mL Erlenmeyer flasks, each containing 100 g of rice and 120 mL of water, and inoculated with strains after high-temperature sterilization; 50 bottles were fermented in each batch, and fermented at 25 °C for 45 days . The fermentation product was extracted with 70% methanol, and then extracted with ethyl acetate, and the ethyl acetate extracted part was concentrated to obtain 210 g of extract.
Embodiment 2
[0060] ------compound separation
[0061] The extract was dissolved in pure methanol with a weight ratio of 2.0 times, and then mixed with 350 g of 100-mesh crude silica gel, and 1.2 kg of 160-mesh silica gel was packed into a column for silica gel column chromatography, and the volume ratio was 1:0, 9:1 , 8:2, 7:3, 6:4, 1:1, 0:1 chloroform-acetone gradient elution, TLC monitoring and merging the same parts to obtain 8 parts, wherein the volume ratio of 9:1 chloroform - The acetone eluted part was separated by Agilent 1100 semi-preparative high performance liquid chromatography, using 60% methanol as mobile phase, Zorbax SB-C18 (21.2 × 250 mm, 5 μ m ) The preparative column is a stationary phase, the flow rate is 12 ml / min, and the detection wavelength of the ultraviolet detector is 254 nm. Each injection is 200 μL, and the chromatographic peaks collected for 22.5 min are evaporated to dryness after repeated accumulation; Dissolved, and then using pure methanol as mobile phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com