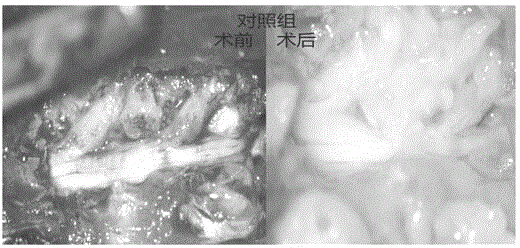
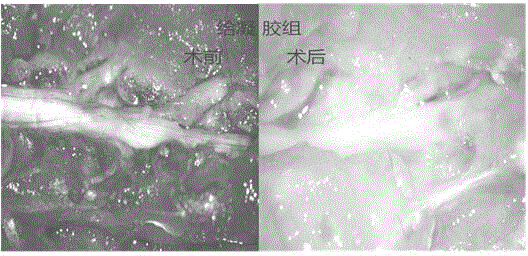
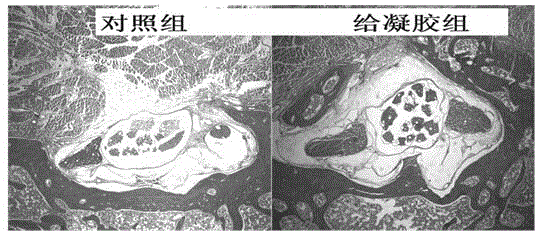
Medicinal injectable anti-adhesive gel and preparation method thereof
An anti-adhesion and gel technology, which is applied in the fields of medical science and surgery, can solve the problems of poor anti-adhesion effect, poor automatic adhesion, and inability to stick wounds, etc., and achieve good biocompatibility and short gelation time , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation of chitosan-sodium glycerophosphate-glutaraldehyde double network rapid response gel (refer to Chinese patent CN103937014A)
[0043] Weigh 2.5 g of chitosan powder (deacetylation degree 98%, molecular weight 100 kDa), dissolve it in 50 mL of 0.1 mol / L hydrochloric acid solution, and obtain a chitosan solution with a mass fraction of 5%. Take out 9mL of this solution, and add 1mL of sodium glycerophosphate solution with a mass fraction of 50% (in which β-sodium glycerophosphate accounts for 90% of the solid powder of sodium glycerophosphate) dropwise in an ice-water bath at 0°C. After the addition is complete, at this time The pH value of the solution is about 7.2, and the solution is stored at 4°C, and this solution is used as solution A. Weigh 0.02g of glutaraldehyde (mass ratio to chitosan in solution A is 0.04:1) and dissolve in 10mL of water for injection, and this solution is used as solution B.
[0044] Put part of liquid A and liquid B int...
Embodiment 2
[0045] Example 2: Preparation of oxidized carboxymethylcellulose and lanine injectable gel
[0046] Weigh 5.0 g of oxidized carboxymethyl cellulose powder (degree of oxidation 20%, molecular weight 100 kDa), dissolve it in 50 mL of PBS buffer solution with pH=7.2, obtain a 10% oxidized carboxymethyl cellulose solution, and take out 2mL was used as liquid A. Weigh 0.02g of lysine (the mass ratio of oxidized carboxymethyl cellulose in solution A is 0.1:1) and dissolve it in 2mL of PBS buffer solution with pH=7.2, and this solution is used as solution B.
[0047] Put liquid A and liquid B into tube A and tube B correspondingly, wherein the liquid volume of tube A and tube B are both 2mL, and the length of tube A and tube B is the same. Then install the AB tube on the sterile double injection device, and at the same time push out the A liquid and the B liquid to mix, the injection time is 10s, after pushing out the mixed liquid, refer to the method of Chinese patent CN103937014A ...
Embodiment 3
[0048] Example 3: Preparation of PEG-NHS active ester and trilysine injectable gel
[0049] Weigh 2.0 g of eight-arm PEG-NHS active ester (NHS substitution degree 95%, single arm molecular weight 10kDa), dissolve it in 50 mL of PBS buffer solution with pH=3.0, and obtain eight-arm PEG-NHS active ester with a mass fraction of 4%. , take out 2mL from it as solution A. Weigh 0.01g of trilysine (the mass ratio of eight-arm PEG-NHS active ester in solution A is 0.125:1) and dissolve it in 2mL of PBS buffer solution with pH=11.2, and this solution is used as solution B.
[0050]Put liquid A and liquid B into tube A and tube B correspondingly, wherein the liquid volume of tube A and tube B are both 2mL, and the length of tube A and tube B is the same. Then install the AB tube on the sterile double injection device, and at the same time push out the A liquid and the B liquid to mix, the injection time is 10s, after pushing out the mixed liquid, refer to the method of Chinese patent C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com