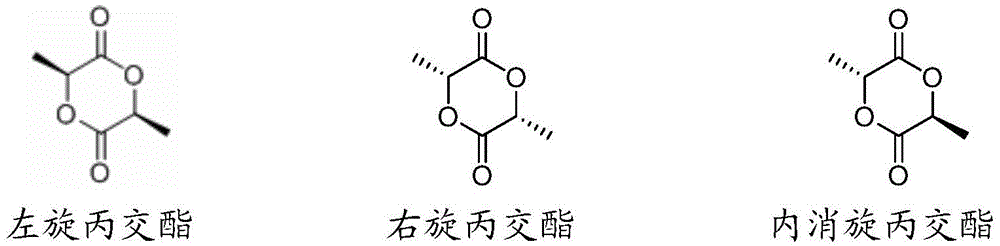
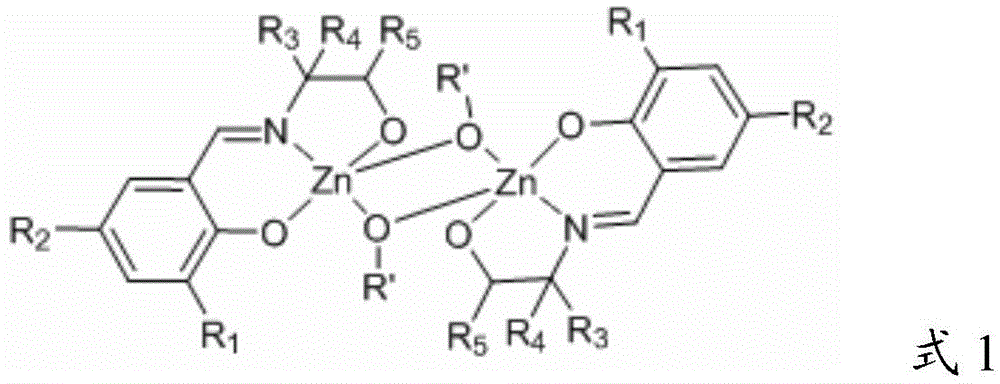
A kind of Schiff base zinc compound, its preparation method and the preparation method of polylactic acid
A technology of zinc and Schiff base compounds, which is applied in the field of polymers and can solve problems such as random linking sequences of chain segments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
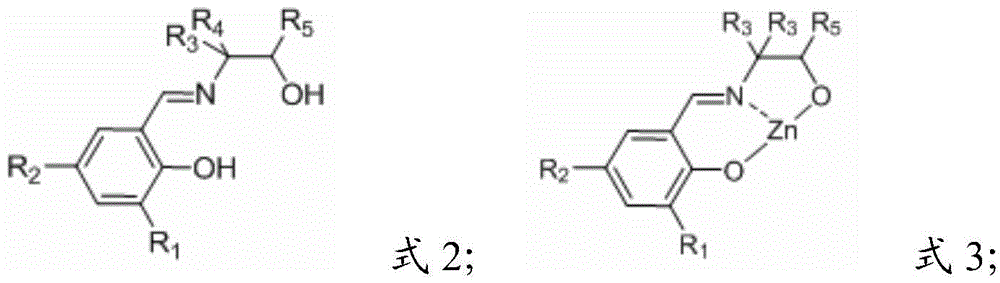
[0045] The present invention also provides a kind of preparation method of Schiff base zinc compound, comprises the following steps:
[0046] A) reacting a Schiff base having a structure shown in formula 2 with an organic zinc compound in a solvent to obtain a Schiff base zinc compound having a structure shown in formula 3;
[0047]
[0048] Among them, R 1 and R 2 independently selected from -H, alkyl, halogen or -NO 2 ;
[0049] R 3 , R 4 and R 5 independently selected from -H or -CH 3 ;
[0050] B) reacting the Schiff base zinc compound having the structure of formula 3 with a hydroxyl-containing compound in a solvent to obtain a Schiff base compound having the structure shown in formula 1;
[0051]
[0052] The R' is an alkoxy group or -OCH 2 One of Ph, the Ph is phenyl.
[0053] In the present invention, the Schiff base with the structure shown in formula 2 is reacted with the organic zinc compound in a solvent to obtain the Schiff base zinc compound with ...
Embodiment 1
[0087]Dissolve 3.05g of amino alcohol in 20mL of ethanol, slowly add dropwise 50mL of ethanol solution containing 6.1g of salicylaldehyde, and reflux the resulting mixed solution for 14h to obtain a reaction solution; rotary evaporation removes most of the solvent in the reaction solution, The obtained reaction product was purified by column chromatography, and the eluent of the column chromatography was n-hexane and ethyl acetate with a volume ratio of 1:1 to obtain a Schiff base.
[0088] The Schiff base that the present invention will obtain carries out nuclear magnetic resonance spectrum 1 HNMR (300.00MHz, CDCl 3 ) analysis, the results show: δ=8.21(s, NCH 1H), 7.24(t, ArH 1H), 7.13(d, ArH 1H), 6.86(d, ArH 1H), 6.79(t, ArH1H), 3.78(t , NCH 2 C 2H),3.60(t,CCH 2 OH 2H). 13 CNMR (100MHz, CDCl 3 )δ=166.67 (NCH), allbenzene ring: 162.12, 132.62, 131.60, 118.44, 118.33, 117.29; 61.67 (NCCOH), 60.95 (COH). This shows that the Schiff base obtained in this embodiment has the s...
Embodiment 2
[0090] Dissolve 3.05g of amino alcohol in 20mL of ethanol, slowly dropwise add 50mL of ethanol solution containing 9.6g of 3,5-dichlorosalicylaldehyde, and reflux the resulting mixed solution for 14h to obtain a reaction solution; rotary evaporation removes the reaction solution. most of the solvent in the solution, and the resulting reaction product was recrystallized with dichloromethane to obtain a Schiff base.
[0091] The present invention carries out the proton nuclear magnetic resonance spectrum analysis to the Schiff base that obtains 1 HNMR (300.00MHz, d 6 -DMSO), the results are as follows:
[0092] δ=14.46(s, OH 1H), 8.51(s, NCH 1H), 7.56(d, ArH 1H), 7.42(d, ArH 1H), 3.67(m, NCH 2 C, CCH 2 OH 4H). 13 CNMR (100MHz,d 6 -DMSO) δ=166.50 (NCH), all ozone ring: 165.02, 133.44, 130.88, 125.48, 116.85, 116.65; 60.15 (COH), 56.25 (NCCOH). This shows that the Schiff base obtained in this embodiment has formula 2 The structure shown, where R 1 and R 2 Simultaneously -C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com