Detection and preparation methods of azilsartan medoxomil impurities
A technology of azilsartan medoxomil and a detection method is applied in the detection and preparation field of azilsartan medoxomil tetramer impurities, can solve the problems such as impurity confirmation and synthesis method that have not been reported in literature, and achieves the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 prepares azilsartan medoxomil
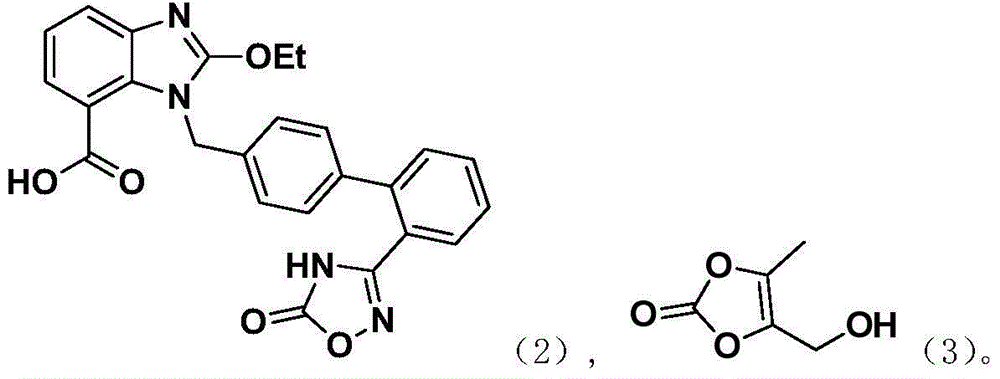
[0041] At 10°C, add azilsartan (8.4g), DMAc (40ml), DMAP (0.5g), TsCl (3.9g), 4-hydroxymethyl-5-methyl-1,3 - After dioxol-2-one (2.4g) was stirred and dissolved, potassium carbonate (2.6g) was added, and after stirring for 3 hours, 0.3N HCl was added dropwise to the reaction solution to adjust the pH to 5, and added dropwise 50ml of acetone / water (1:1), precipitated a large amount of solids, kept warm for 1h, filtered to obtain a wet product, then added acetone (20ml) to make a slurry, filtered, and vacuum dried at 50°C for 10h to obtain 1.8g of azilsartan medoxomil, HPLC purity 96.8% .
Embodiment 2L
[0042] Embodiment 2LC-MS detection method
[0043] The azilsartan medoxomil prepared in Example 1 was dissolved in the diluent DMF,
[0044] Chromatographic column: Agilent Eclipse XDB C184.6x 250mm, 5um;
[0045] Mobile phase: A phase: 10Mm ammonium acetate buffer salt (pH3.5), B phase: acetonitrile;
[0046] Column temperature: 30°C;
[0047] Running time: 60min, post-running time: 8min;
[0048] Elution gradient:
[0049] Time(min)
A%
B%
0
55
45
8
55
45
18
40
60
28
10
90
60
10
90
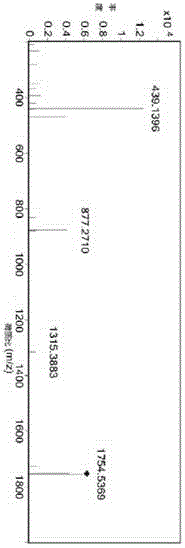
[0050] The detector is a quadrupole time-of-flight mass spectrometer (Q-TOF).
[0051]ESI positive polarity, automatic MS / MS mode; Capillary voltage: 3.5Kv; Nebulizer pressure: 45psi; Dry gas (nitrogen) flow rate: 12L / min;
[0052] Drying gas temperature: 350°C.
[0053] Q-TOF full scan results show that m / z1753.5375 and m / z1791.4935 have unknown impurities [M+H] + Ions and [M+K] + ions, in...
Embodiment 3
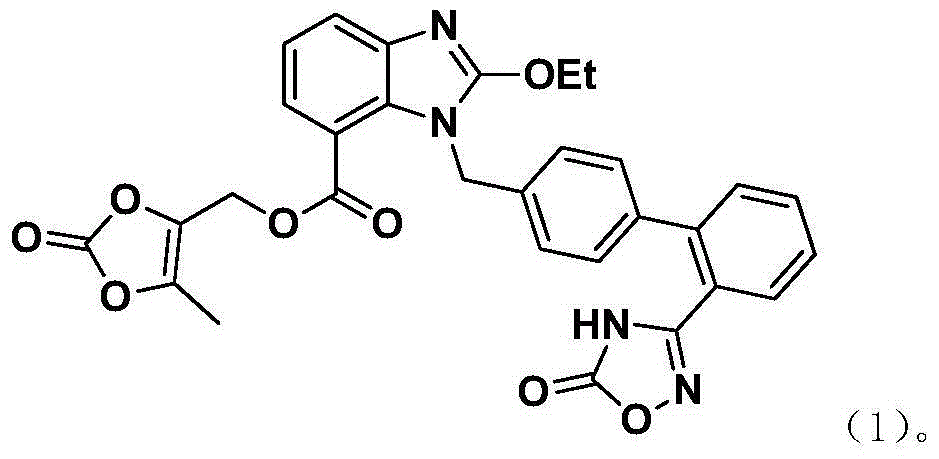
[0054] Embodiment 3: the synthesis of azilsartan tetramer impurity
[0055] At 10°C, Azilsartan (8.4g), DMAc (50ml), DMAP (0.5g), TsCl (3.9g) were added successively to the reaction vessel and stirred to dissolve, then triethylamine (6.5g) was slowly added dropwise, After insulated and stirred for 3 hours, 0.3N HCl was added dropwise to the reaction solution to adjust the pH to 4-6. Add 50ml of acetone / water (1:1) dropwise, a large amount of solids precipitate out, keep warm for 1h, filter to obtain a wet product, then add acetone (20ml) for slurry, filter, and vacuum dry at 50°C for 10h to obtain 5.6g of pure tetramer (HPLC purity : 99.75%), retention time: 38.36min, relative retention time: 2.24min.
[0056] The target unknown impurity prepared is subjected to NMR experiment:
[0057] Instrument: Bruker AVANCE AV 400 superconducting pulse Fourier transform nuclear magnetic resonance spectrometer,
[0058] Analysis method: JY / T 007-1996 Superconducting Pulse Fourier Transf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com